
ِArticle topic: Hydrocephalus
Author: Leen Mohammad AlHadidi
Editor: Haneen A. Banihani, Haneen Al-Abdallat
Final Reviewer: Zain Alsaddi
Keywords: Hydrocephalus, Acquired Hydrocephalus, Communicating Hydrocephalus, Obstructive Hydrocephalus, Congenital Hydrocephalus, Neuroinflammation.
Introduction
“Hydro” and “Kephal” (which means “water on the brain”) as described by Hippocrates, Galen, and early and medieval Arabian physicians, were believed to have been caused by extracerebral accumulation of water.1
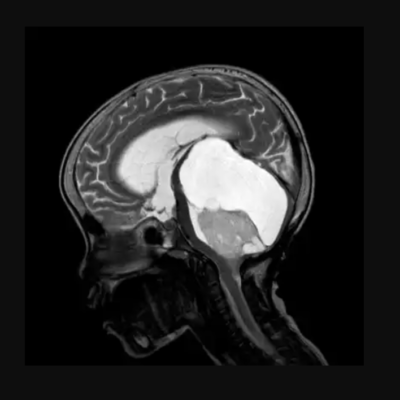
Hydrocephalus is an abnormal accumulation of the cerebrospinal fluid (CSF) within the cavities of the brain. This is often due to an imbalance in the dynamic of CSF production by choroid plexuses and reabsorption by arachnoid granulation. The accumulated fluid leads to an enlargement of the ventricles, causing an elevation in intracranial pressure (ICP). These enlarged ventricles exert a “mass effect,” compressing neighboring brain tissue and ultimately giving rise to interstitial edema, ischemia, oxidative stress, and resulting in overall cortical tissue injury, with a predilection for the middle temporal and middle frontal gyri.2,3,4,5
While there is no known specific cause for hydrocephalus, most cases can result from bleeding, viral infections, or meningitis, and rarely by congenital defects. This condition can be classified into 4 main types: Normal pressure hydrocephalus, communicating hydrocephalus, non-communicating or obstructive hydrocephalus, and hydrocephalus ex-vacuo.6
Epidemiology
The worldwide prevalence of hydrocephalus stands at 84.7 cases per 100,000 individuals, exhibiting notable variations across age groups, with the elderly experiencing the highest prevalence. Over 11 years, the mean annual incidence of congenital hydrocephalus was recorded at 49.5 cases per 100,000 for isolated hydrocephalus. Intriguingly, lower incidence rates were observed in high-income countries. Notably, there was no significant disparity in the mean incidence of congenital hydrocephalus between countries with and without mandatory folate fortification. Furthermore, the prevalence appears to be consistent among both males and females.7,8
Post-infectious hydrocephalus (PIH) is the predominant form of acquired hydrocephalus in resource-poor countries, and post-hemorrhagic hydrocephalus (PHH) is the most common cause of acquired pediatric hydrocephalus in resource-rich countries. Approximately, 25-30% of neonates who experience severe Intraventricular hemorrhage will develop post-hemorrhagic hydrocephalus (PHH).9,10
The cerebrospinal fluid (CSF)
The CSF is a clear proteinaceous fluid, roughly estimated to be 150ml in adults, and is distributed between compartments as follows: 125ml in the subarachnoid space and 25ml within the ventricles, with marked interindividual variations. It plays an essential role in the homeostasis of the interstitial fluid of the brain parenchyma and the regulation of neuronal functioning.11
The ependymal cells of the choroid plexuses are specialized cells located in the lateral and fourth ventricles of the ventricular system and are responsible for the production of the CSF. The extra-choroidal secretions, which are derived from extracellular fluid and cerebral capillaries, across the blood-brain barrier also contribute to CSF production. Once the CSF is done circulating through the ventricular system, it gets reabsorbed back into circulation by the Arachnoid Granulations and may also be significantly absorbed by the cervical lymphatics. Throughout this process, the CSF gets renewed 4-5 times per 24 hours in young adults.8,11,12
CSF circulation
The CSF circulates from sites of secretion to sites of absorption in a unidirectional rostro-caudal flow manner within the ventricular cavities and in a multidirectional flow in the subarachnoid spaces. Structures called “ciliated ependyma” beat in synchrony to assure a unidirectional flow in the ventricular system.11
CSF circulation follows the Bulk Flow Model, which states that the flow is pulsatile and in correspondence with the systolic pulse wave in the choroidal arteries. The CSF travels from the lateral ventricles through the right and left interventricular foramina of Monro to the 3rd ventricle, and then to the 4th ventricle via the cerebral aqueduct of Sylvius and finally to the subarachnoid spaces via the foramen of Magendie medially and foramen of Lushka laterally.11,12,13
According to the Model, hydrocephalus is the result of a physical or a functional obstruction that may lie within the ventricular system, the subarachnoid spaces, or the venous sinuses.13
Classification
Hydrocephalus can be classified as Obstructive vs. Communicating Hydrocephalus, Acquired vs. Developmental Hydrocephalus, and Syndromic vs. non-syndromic Hydrocephalus.2
A “Multi-categorical Hydrocephalus Classification” (Mc HC), a recently reported classification of hydrocephalus, was developed to provide comprehensive coverage of the entire aspects of hydrocephalus. Other categories, such as intracranial pressure patterns and specific clinical features, have been used to classify hydrocephalus.14,15
The most used classification of hydrocephalus is Communicating vs. Obstructive, and it was the result of an experiment conducted in the 1800s and early 1900s by the Walter Dandy dog model of obstructive hydrocephalus. 16
Communicating Hydrocephalus is caused by impaired absorption of the CSF, which may be a result of multiple causes including hemorrhage, inflammation, tumors, or elevated pressure within the venous sinuses. Obstructive Hydrocephalus, on the other hand, is an active dilatation of the ventricles, that is caused by structural blockage of CSF circulation within the ventricular system to its point of absorption into the systemic circulation. 8,13,17
Etiologies of Hydrocephalus
Hydrocephalus can result from an extrinsic event that acts upon a structurally normal brain, or it can occur either in the setting of an identifiable clinical/molecular syndrome or be purely idiopathic.2
Acquired Hydrocephalus
Also referred to as “secondary hydrocephalus”, is caused by various etiologies like brain tumors, trauma to the head, infection, hemorrhage, stroke, and others. Intraventricular hemorrhage remains the predominant cause of hydrocephalus in premature infants.13,18
Post-hemorrhagic hydrocephalus (PHH) and post-infectious hydrocephalus (PIH) are two other common forms of hydrocephalus worldwide. PHH occurs primarily in preterm neonates with a very low birth weight (< 1500 grams), in whom the condition is secondary to germinal matrix hemorrhage (a rapid cell proliferation area located between the wall of the lateral ventricle and the caudate nucleus and still present until approximately 34 weeks gestation). PHH is also a common cause of hydrocephalus in adults. It is often associated with intraventricular hemorrhage resulting from hypertension, aneurysmal rupture, or traumatic brain injury.9
PIH, on the other hand, is the most common cause of pediatric hydrocephalus worldwide and is most prevalent in Africa, Latin America, and Southeast Asia. PIH in infants can result from either antenatal infections or postnatal infections. Some of the common causative organisms of bacterial sepsis include Escherichia coli, Streptococcus agalactiae, and Listeria monocytogenes. In resource-rich countries, prenatal infection is often caused by Toxoplasma gondii and cytomegalovirus. A case study, published in 2023, suggested that acute hydrocephalus may be a potential complication of recent COVID-19 infection.9,19,20
Infant tumor-related hydrocephalus (iTRH) is found in over 70% of new diagnoses overall and in over 90% of infants diagnosed with infratentorial neoplasms. iTRH may be related to direct obstruction of CSF pathways, shedding of protein or cells leading to dysfunctional subarachnoid, tumor-related CSF production, or, most frequently, a combination of these mechanisms altogether. In patients with malignant CNS tumors, hydrocephalus was discovered in 62%, however, its frequency did not depend on the degree of malignancy.21,22
Congenital hydrocephalus
Congenital hydrocephalus happens due to genetic factors, congenital malformations or birth defects, congenital brain tumors, and intracerebral hemorrhages. It may present as a part of a syndrome (syndromic) or alone (non-syndromic).
Spina bifida is the most common congenital abnormality of the CNS that is compatible with life, it entails a problem with neural tube closure. Maternal folic acid deficiency has been implicated with a 2-8-fold increased risk of babies with this malformation. While only 10% of neonates have clinically apparent hydrocephalus at birth, the incidence sharply increases within the first week and hydrocephalus manifests in up to 85% of cases.23
A study held in Ethiopia shows that alcohol use and inadequate supplementation of iron and folic acid during pregnancy were significantly associated with the development of congenital hydrocephalus. Other factors were maternal exposure to typhus and typhoid and the use of antibiotics during early pregnancy. 5
Encephaloceles are rare congenital malformations of the CNS in which there is herniation of intracranial contents through a defect in the skull. In cases of occipital encephalocele, hydrocephalus is a common complication and it occurs in 60-90% of cases. 24
Conditions that are associated with hydrocephalus
1- Chiari II Malformation, also known as Arnold-Chiari malformation, is a relatively common congenital anomaly believed to arise due to in-utero malformation of the cranial and spinal structures. This condition leads to the displacement of the cerebellum, medulla, and fourth ventricle through the foramina magnum. According to “Rat fetus models with induced Arnold Chiari malformation”, hydrocephalus is caused by plugging the foramen magnum, and is not a primary disorder. 25,26
2- Dandy-Walker malformation is a posterior fossa anomaly stemming from developmental abnormalities impacting the midbrain roof. This condition results in the agenesis or hypoplasia of the vermis and cystic enlargement of the fourth ventricle, leading to an upward displacement of the tentorium and torcula. Although typically a sporadic disorder, environmental factors, such as congenital infections, have been identified as potential contributors. In a case series of 42 patients with this malformation, all had hydrocephalus at the time of diagnosis. 27,28
3- Aqueductal stenosis represents another possible etiology of hydrocephalus. This condition may arise idiopathically, genetically, or as a consequence of secondary pathologies such as infection, hemorrhage, or malformations within the central nervous system. It is characterized by an obstruction occurring between the third and fourth ventricles, ultimately leading to the manifestation of non-communicating hydrocephalus. The most common genetic form associated with aqueductal stenosis is the X-linked L1 syndrome. 29
Pathogenesis of hydrocephalus
Direct blockage of CSF flow
Several case series were conducted to identify the obstructive mechanisms associated with PHH (Post-Hemorrhagic Hydrocephalus) and PIH (Post-Infectious Hydrocephalus).
In the context of PHH, these case series suggested that blood and its breakdown products could acutely obstruct narrow CSF passages, as seen in cerebral aqueducts. Alternatively, some proposed another form of PHH known as “tetra-ventricular PHH,” characterized by obstruction of the leptomeninges due to fibrous thickening that impedes the outflow from the fourth ventricle. For PIH, the obstruction is often attributed to inflammatory debris resulting from infection, which blocks either the cerebral aqueduct or the outlet of the fourth ventricle, particularly in patients with basal meningitis.9,19
While obstructive mechanisms were a proven causative factor in both PHH and PIH, many patients diagnosed with these conditions displayed no evidence of physical obstruction to the CSF flow in the ventricular and subarachnoid spaces. Hence, other possible causes were proposed.9
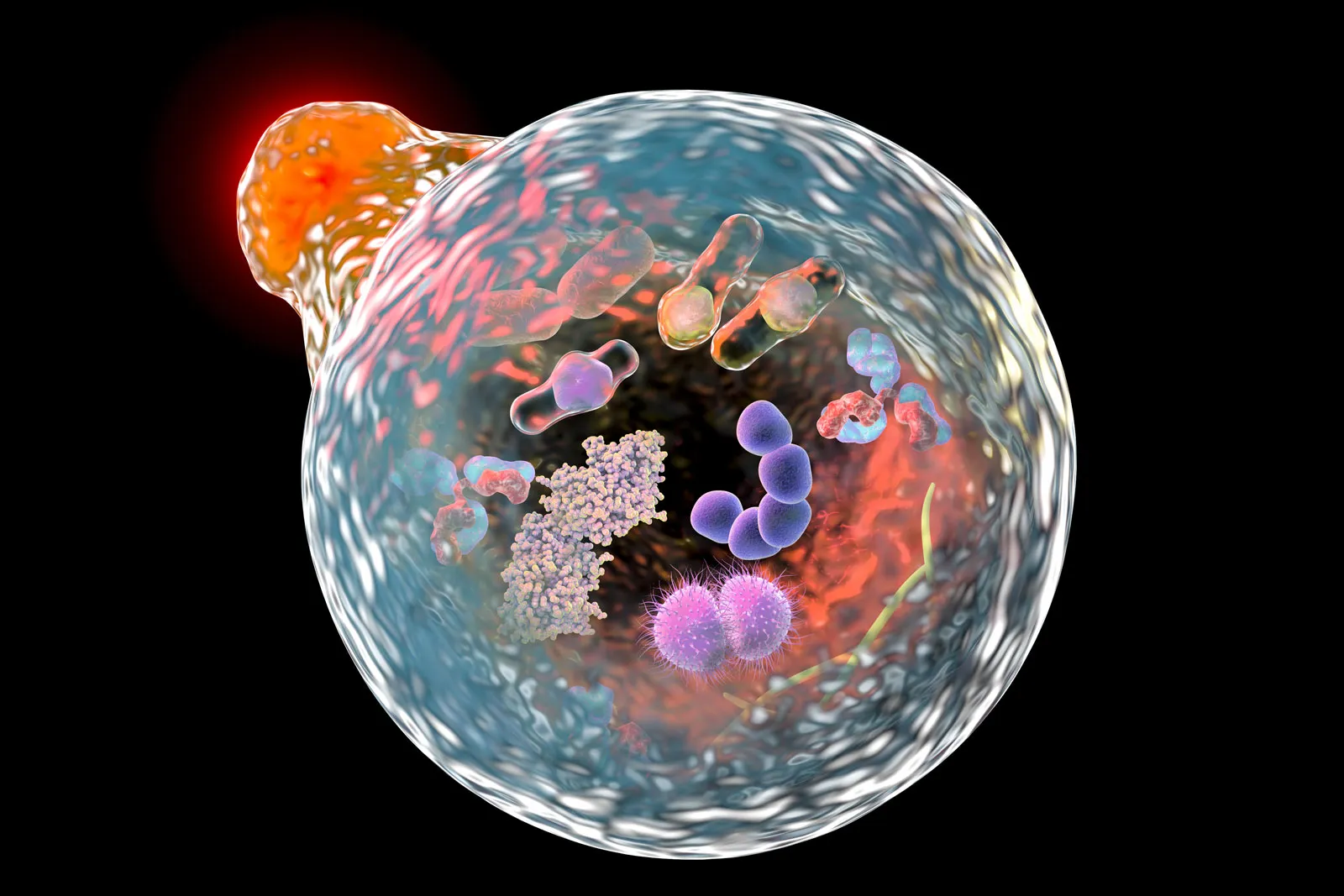
Neuroinflammation
The choroid plexus epithelium (CPe) is one of the first brain structures to encounter extravasated blood after intraventricular hemorrhage. Structural and cellular damage to the choroid plexus was investigated following Intraventricular Hemorrhage in preterm rabbit pups. The resulting data showed that extracellular hemoglobin is a causal inducer of inflammation, apoptosis, and cytotoxicity in the immature brain. In animal models, intracerebroventricular injection of blood metabolites causes CPe inflammation and hydrocephalus.30,33
Evidence has shown that PHH and PIH may be a result of an inflammatory reaction that impairs normal ependymal and choroid plexus function. According to the model proposed by Dr. Kahle and colleagues, in the first seven days after an infection or a hemorrhage, CSF hypersecretion occurs and is then followed by chronic inflammation. This event led to scar formation and subsequently resulted in CSF malabsorption (hypersecretion of CSF acutely, then malabsorption in the chronic phase).13,30
The lipopolysaccharide present in PIH and the blood breakdown products in PHH initiate remarkably similar TLR4-dependent immune responses at the choroid plexus–CSF interface. This leads to the occurrence of a “CSF cytokine storm,” which, in turn, recruits macrophages from the periphery and activates border-associated choroid plexus macrophages. This activation results in an augmented production of cerebrospinal fluid (CSF) from choroid plexus epithelial cells through the phospho-activation of the TNF-receptor-associated kinase (SPAK).32
In infants and adults who have had an infection or brain hemorrhage, levels of IL-6, IL-4, TNF-α, TGF-β1 and other inflammatory markers in the CSF and the peripheral blood correlate with the possibility of developing hydrocephalus, and with the severity of the condition. Also, hydrocephalus was associated with a persistent inflammatory response and raised protein concentrations and other inflammatory markers in the CSF. A study comparing hydrocephalic brains with controlled non-hydrocephalic ones showed that hydrocephalic brain tissue had more ependymal and supra-ependymal macrophages. 9,30,34
A different trial was aimed at studying the association between hydrocephalus and neuroinflammation. During the trial, hydrocephalus was induced in 35-day-old female pigs. A significant increase in astrocytes and microglia in the periventricular white matter and high levels of inflammatory interleukins IL-6 and IL-8 in the CSF were detected. 35
In another clinical trial employing a randomized, double-blinded, and placebo-controlled design, 545 adults with tuberculous meningitis were administered the corticosteroid dexamethasone. The trial revealed a reduction in the frequency of hydrocephalus in individuals who received dexamethasone compared to those who received a placebo. Dexamethasone, when used in high doses, is associated with a decreased frequency of hydrocephalus following aneurysmal subarachnoid hemorrhage as well. 30,36
Iron also has a distinct role in hydrocephalus. Ependymal cells normally take up iron from the CSF and prevent its diffusion to the rest of the brain; consequently, any damage to the ependymal cells will lead to iron accumulation in the brain parenchyma, bilateral enlargement of the lateral ventricles, and hippocampal brain tissue loss.31,37,38
Another crucial factor triggering inflammation is thrombin, a serine protease responsible for initiating blood clotting by cleaving fibrinogen into fibrin. Thrombin becomes activated immediately after hemorrhage as part of the coagulation cascade. It induces inflammation through ischemia resulting from thrombus generation, either directly via protease-activated receptors (PAR) or indirectly through leukocyte recruitment. Experimental evidence, such as the injection of heparinized blood into the ventricles, demonstrated a reduced risk of hydrocephalus compared to whole blood. This suggests that a component of the coagulation cascade, likely thrombin, plays a pivotal role in the development of post-hemorrhagic hydrocephalus (PHH). 10
Conclusion
Hydrocephalus, characterized by abnormal accumulation of cerebrospinal fluid (CSF) in the brain, poses challenges in understanding its varied etiologies and pathogenic processes. This condition results from imbalances in CSF production and reabsorption, causing enlarged ventricles, elevated intracranial pressure, and subsequent tissue injury. Pathogenesis involves direct blockage of CSF flow, with neuroinflammation contributing to choroid plexus damage, scar formation, and malabsorption, revealing the intricate nature of hydrocephalus that necessitates a nuanced understanding for effective management.
The Multi-categorical Hydrocephalus Classification encompasses normal pressure, communicating, non-communicating, and hydrocephalus ex-vacuo, highlighting the complexity of this condition that affects various age groups, with post-infectious hydrocephalus predominant in resource-poor countries and post-hemorrhagic hydrocephalus common in neonates.
Etiologies range from acquired factors like trauma and tumors to congenital causes, impacting individuals worldwide. While specific causes remain elusive, hydrocephalus is often linked to factors such as bleeding, viral infections, or meningitis.
References...







Excellent
Very important article