Article topic: Cerebellum: The Little Brain
Author: Abed Dhadil
Editors: Yara Alswaiti, Rawan Hamamreh
Reviewer: Omar B. Sanduka
Overview
The cerebellum is a crucial part of the brain located at the posterior region of the head. Its primary functions include postural adjustment and the maintenance of balance [1]. Moreover, the cerebellum plays a pivotal role in motor skills and functions. This process typically involves a shift from “controlled” to “automatic” processing, wherein movements that initially necessitate problem-solving and focused attention become progressively more efficient, stereotypical, less responsive to real-time feedback, and notably demand reduced attention [2]. Across all vertebrates, the cerebellum develops from two bilaterally symmetrical anlagen, which are dorsally positioned at the anterior end of the rhombencephalon [3]. Dysfunction in the cerebellum is evident in various developmental disorders, including autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and developmental dyslexia. Furthermore, damage to the cerebellum during early development can have enduring impacts on motor skills, cognition, and emotional regulation [4].
Introduction
The cerebellar cortex consists of several distinct components, including granule cells, Purkinje cells, and two types of inhibitory interneurons known as Golgi cells and stellate/basket cells. This cortex receives input from three types of extracerebellar afferents: mossy fibers, climbing fibers (both of which are excitatory), as well as diffusely organized monoaminergic and cholinergic afferents.
The output of the cerebellar cortex follows a unique pattern organized into parallel longitudinal zones. These zones can span one or more lobules, with some extending across the entire rostrocaudal length of the cerebellum. It’s important to note that within the vermis and hemispheres, these zones remain parallel to the long axis of the folial chains [5] (see Figure 1 for reference).
![Figure 1: Striped distribution pattern of Purkinje cells of different birthdates in the mouse cerebellar cortex [6]](https://neuropedia.net/wp-content/uploads/2023/07/figrue-1-1-300x88.jpg)
Figure 1: Striped distribution pattern of Purkinje cells of different birthdates in the mouse cerebellar cortex [6]
The primary function of the cerebellum encompasses motor control, which includes regulating movement and balance and maintaining kinesthetic homeostasis. Additionally, the cerebellum has a secondary, albeit minor, non-motor function. Mossy fibers, originating from neurons in the brainstem, synapse onto granule cells. These granule cells send out axons referred to as parallel fibers, which ascend into the higher layers and levels of the cerebellar cortex. There, they branch and establish excitatory connections with numerous Purkinje cells, often numbering in the thousands. Some of the parallel fibers also synapse on basket or stellate cells, which in turn inhibit the Purkinje cells. Additionally, certain parallel fibers synapse on the dendrites of Golgi cells, which makes inhibitory connections back to the granule cells. Mossy fiber collaterals project onto neurons located in the deep cerebellar nuclei. Climbing fibers originate in the inferior olive and ascend to the cerebellar cortex. There, they wind around one or two Purkinje cells, forming thousands of synaptic contacts with them. Climbing fibers also send their collaterals directly to neurons in the deep cerebellar nuclei [7].
Psychological function of the cerebellum
The cerebellum is thought to play a significant role in adapting general problem-solving circuitry to specific contextual demands. Prefrontal cortical regions, which are involved in complex problem-solving tasks, may develop a generalized cognitive program that is then refined through interaction with the cerebellum. This collaboration between brain regions represents a precise and coherent solution to cognitive challenges. This function can be described as attentional, particularly in the context of certain tasks that require the specific identification of relevant stimuli for processing. Error-correcting feedback mechanisms are crucial for organizing this coherence, potentially acting as a filter to determine which components of available systems are integrated into the coherent solution. For instance, if a critical stimulus is omitted from the motor or cognitive program, error correction mechanisms will ensure the inclusion of all necessary circuitry in the coherent solution. Furthermore, the exclusive inhibitory function in the essential cortico-nuclear connection can be likened to a circuit breaker, serving as the ultimate stabilization device. It facilitates the dissolution of coherence when necessary, allowing for transitions into new behavioral modes. This includes shifts from feedback to feedforward movement phases, the release of a conditioned eye blink, or preparation for new stimuli in cognitive tasks. In light of these observations, we propose that the cerebellum actively participates in a range of psychological functions, including attention, timing, and learning [7]. Figure (2)
![Figure 2: Anatomical and functional organization of the cerebellum [8]](https://neuropedia.net/wp-content/uploads/2023/07/Anatomical-and-functional-organization-of-the-cerebellum-A-The-cellular-architecture-245x300.png)
Figure 2: Anatomical and functional organization of the cerebellum [8]
Grey matter –On the surface of the cerebellum. It is tightly folded, forming the cerebellar cortex. The gray matter of the cortex is divided into three layers: an external – the molecular layer; a middle – the Purkinje cell layer; and an internal – the granular layer. The molecular layer contains two types of neurons: the outer stellate cell and the inner basket cell.
White matter – Located underneath the cerebellar cortex. There are four nuclei embedded in the white matter (the dentate, emboliform, globose, and fastigi nuclei). (Figure 3)
![Figure 3: Nuclei of the cerebellum. [9]](https://neuropedia.net/wp-content/uploads/2023/07/image452-300x194.jpg)
Figure 3: Nuclei of the cerebellum. [9]
The dentate nucleus, positioned as the most lateral of the cerebellar nuclei and distinguished by its richness in iron, resides in close proximity to both the cerebellar vermis and the roof of the fourth ventricle. Its pivotal role encompasses the regulation of fine control over voluntary motor movements, cognitive processes, language, and various sensory functions.
The dentate nucleus has two parts: the dorsal (motor) domain which has connections with cortical motor areas that regulate motor functions, and the ventral (non-motor) domain which has links with the cortical association areas that control major cognitive functions.
Input to the dentate nucleus
Afferent proprioceptive information synapses with dentate nucleus neurons through the spinocerebellar tract through the inferior cerebellar peduncle. Both the premotor cortex and supplementary cortex neurons also synapse with the dentate nucleus. [10]
Output to the thalamus
Utilizing the dentate-thalamic tract, the dentate nucleus sends output signals via the ipsilateral superior cerebral peduncle and then decussates to synapse the contralateral ventrolateral (VL) thalamic nucleus. VL neurons then send fibers to the precentral gyrus (primary motor cortex; M1), premotor cortex, prefrontal gyri, posterior parietal areas, and the basal ganglia – specifically the striatum and the substantia nigra. [10]
Myoclonic triangle
The myoclonic triangle, also known as the triangle of Guillain-Mollaret, comprises the dentate nucleus, the red nucleus, and the inferior olivary nucleus (ION) located within the medulla oblongata. This intricate network plays a pivotal role in the control of fine voluntary movements. Through the dentate-rural tract, the dentate nucleus sends fibers via the superior cerebellar peduncle to the contralateral red nucleus. Parvocellular red nucleus axons, in turn, establish synapses with the ipsilateral ION through the central tegmental tract. Axons originating from the ION traverse through the inferior cerebral peduncle as climbing fibers, ultimately reaching the contralateral cerebellar cortex, where they synapse on Purkinje cells. Subsequently, Purkinje cells complete the loop by synapsing with the ipsilateral dentate nucleus. [10]
Cerebellar Purkinje cells
Cerebellar Purkinje cells, which are GABA-ergic neurons located in the cerebellar cortex, synapse on deep cerebellar nuclei such as the dentate nucleus. They have an inhibitory role on the deep cerebellar nuclei. [10]
![Figure 4: Dentate nucleus, case courtesy of Craig Hacking. [11]](https://neuropedia.net/wp-content/uploads/2023/07/Gray707_jumbo-300x239.jpeg)
Figure 4: Dentate nucleus, case courtesy of Craig Hacking. [11]
The caudal fastigial nuclei (cFN) are the output nuclei by which the medio-posterior cerebellum influences the production of the visual saccades. Unilateral inactivation of the caudal fastigial nucleus results in a reduced accuracy of saccades directed toward moving targets. [12] Similar to saccades aimed at stationary targets, interceptive saccades exhibit a hypometric pattern when directed towards the contralesional side and a hypermetric pattern when directed towards the ipsilesional side. The dysmetria depends on target velocity, but the use of accelerating or decelerating targets reveals that velocity is not the crucial parameter. [12]
The major cerebellar circuit
The ultimate destination of afferent pathways to the cerebellar cortex is a distinctive cell type called Purkinje cell. However, the input from the cerebral cortex to the Purkinje cells is indirect. Neurons in the pontine nuclei receive a projection from the cerebral cortex and then relay the information to the contralateral cerebellar cortex. The axons from the pontine nuclei and other sources are called mossy fibers (because of the appearance of their synaptic terminals). Mossy fibers then synapse on granule cells in the granule cell layer of the cerebellar cortex. The cerebellar granule cells are widely held to be the most abundant class of neurons in the brain. They give rise to specialized axons called parallel fibers that ascend to the molecular layer (external or outermost layer) of the cerebellar cortex. The parallel fibers bifurcate in the molecular layer to form T-shaped branches that relay information via excitatory synapses onto the dendritic spines of the Purkinje cells. [13]
Cerebellar dysfunction
Cerebellar dysfunction is associated with many manifestations and alternations in cerebellar functions including, decreased muscle tone (hypotonia), eye problems, including double vision and involuntary eye movements (nystagmus), poor muscle coordination in the limbs (ataxia). As previously mentioned, it appears to have a critical role in autism spectrum disorder (ASD).
The role of cerebellar dysfunction in ASD patients
In ASD patients, there are observable increased connections between the supra-modal regions of the cerebellum and the sensorimotor cortex, as well as reciprocal connections. Furthermore, research has subdivided the temporoparietal junction, a region crucial for social cognition, into eleven subdivisions. It was noted that ASD patients display a decrease in connectivity between the right dorsal temporoparietal junction and the left cerebellar Crus II.
However, it is essential to acknowledge that different studies have reported varying findings. One study, for instance, observed an increase in functional connectivity between the left inferior parietal lobule and the right Crus I in ASD patients. These diverse outcomes underscore the ongoing debate regarding which specific regions of the cerebellum and cerebral cortex play a significant role in ASD etiology.
Additional studies have highlighted decreased resting state connectivity between the left cerebellar dentate nucleus and cortical regions associated with the theory of mind and mode networks in adults with ASD. Furthermore, hyperconnectivity was detected between the left dentate nucleus and supra-modal regions of the cerebellum in ASD patients. Lastly, one study revealed a significant decrease in the volume of the right Crus II and a reduction in connectivity between the right Crus II and the frontal, temporal, and parietal regions in individuals with ASD. [14]
Role of genetics in cerebellar dysfunction and ASD
The majority of ASD cases result from multiple genetic mutations and exhibit a high degree of heritability. To investigate the cognitive, emotional, and motor dysfunctions associated with this disorder, the creation of animal models, such as rodents, with genetic mutations linked to ASD becomes an appealing avenue of study.
Utilizing mouse models, which enables the manipulation of genetic factors, proves to be an effective method for elucidating the impacts of both distal and proximal etiological influences on cerebellar function and cerebro-cerebellar circuitry. The insights gained from mouse models serve to validate and expand our comprehension of the neurochemical and behavioral consequences brought about by genetic changes. Furthermore, these models aid in the search for biomarkers of ASD.
In particular, researchers can leverage the advantages offered by mouse genetics to explore whether cerebellar dysfunction alone can give rise to ASD-related behaviors.
![Figure 5: Brain scan of an ASD patient by Devika G. Bansal. [15]](https://neuropedia.net/wp-content/uploads/2023/07/brain-scan-300x199.jpg)
Figure 5: Brain scan of an ASD patient by Devika G. Bansal. [15]
Autosomal dominant cerebellar ataxias (ADCA) have traditionally been classified into ADCA types I, II, and III, as well as episodic ataxia, based on clinical criteria.
ADCA type I (linked to gait and position): In ADCA type I, individuals experience cerebellar ataxia affecting both gait and limb function. This presentation may vary and is often accompanied by a range of additional features such as supranuclear ophthalmoplegia, slow saccades, optic atrophy, pyramidal and extrapyramidal symptoms (e.g., parkinsonism, dystonia, chorea), sphincteric disturbances, mild to moderate dementia, amyotrophy, and peripheral neuropathy. [16]
ADCA type II shares the same clinical phenotype as ADCA type I but is further associated with macular degeneration. ADCA type III manifests as late-onset cerebellar atrophy.
In recent years, ADCA has undergone subdivision into various types known as spinocerebellar ataxias (SCAs), encompassing numbers one through sixteen. This classification is based on the specific genes or chromosomal loci associated with the disease. The different forms of ADCA have several clinical and genetic features: (a) onset in adulthood; (b) occurrence of anticipation, i.e. earlier age at onset and increasing severity of the disease in subsequent generations; (c) cerebellar atrophy or olivopontocerebellar atrophy seen in brain imaging studies; (d) molecular defects in the cloned genes consisting of an expansion of trinucleotide or pentanucleotide repeats; and (e) presence of an inverse correlation between expansion size and the degree of clinical involvement. The majority of spinocerebellar ataxias, and the form named dentatorubral-pallidoluysian atrophy (DRPLA), are due to pathological expansions of a CAG-repeated sequence within the coding region, the part of the gene that will be eventually transcribed and translated into protein, of the corresponding genes. The mutated proteins, which carry the elongated polyglutamine tract, supposedly gain a toxic function, leading to neuronal cell death and neurodegeneration. [16]
![Figure 6: The MR (T1) brain (A) sagittal, (B) axial in a 54-year-old African-American male with genetically confirmed SCA2 who presented with cerebellar ataxia, dysarthria, slowed saccades, and peripheral neuropathy demonstrates cerebellar and brainstem atrophy. [17]](https://neuropedia.net/wp-content/uploads/2023/07/13023_2009_Article_291_Fig1_HTML-300x134.png)
Figure 6: The MR (T1) brain (A) sagittal, (B) axial in a 54-year-old African-American male with genetically confirmed SCA2 who presented with cerebellar ataxia, dysarthria, slowed saccades, and peripheral neuropathy demonstrates cerebellar and brainstem atrophy. [17]
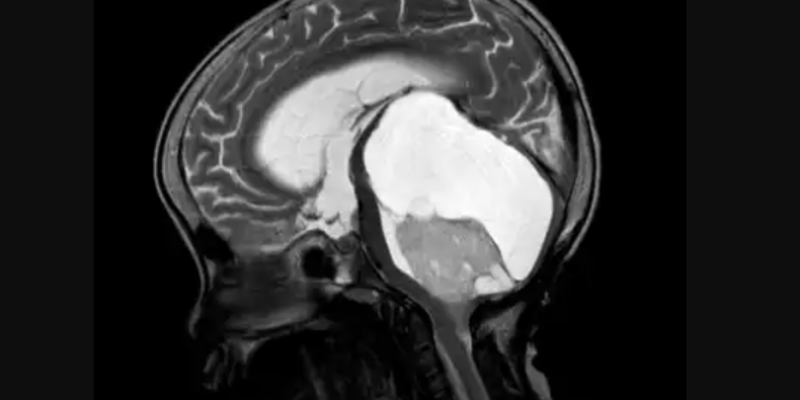
The cerebellum is the most common site of presentation of CNS tumors in children but it is extremely rare in adults. Children usually present with acute symptoms related to increased intracranial pressure that require urgent surgical intervention. The differential diagnosis is broad and includes a variety of benign and malignant entities. [18]
- Pediatric low-grade gliomas (PLGGs)
Benign tumors of glial origin can arise anywhere in the central nervous system, most commonly (one-third of tumors) in the cerebellum. The most common histology is a pilocytic astrocytoma, characterized by the presence of cells with bipolar “hair-like” processes, low mitotic index, and the presence of Rosenthal fibers on histology. Though PLGGs are often associated with genetic predisposition syndromes – most notably neurofibromatosis type 1 (15%) – the lesions in children with neurofibromatosis type 1 are located in the optic pathway, while cerebellar tumors are uncommon. [18]
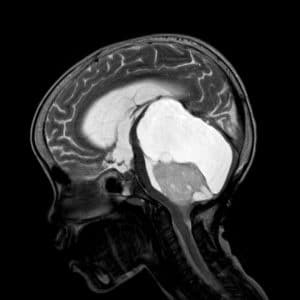
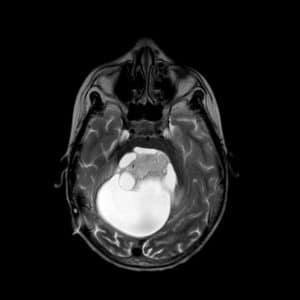
Figure 7: Sagittal & axial sections of a pilocytic astrocytoma. Case courtesy of Abiola Ayodele. [19] - Dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos-disease)
Lhermitte-Duclos-disease is a slow-growing cerebellar mass. It is a matter of debate whether it represents a neoplasm or a hamartoma. Histologically, the lesion comprises dysplastic ganglion cells localized predominantly in the internal granule cell layer. Imaging characteristics may be enough for diagnosis, due to a typical striated pattern on T2-weighted sequences with a lack of contrast enhancement. It is associated with Cowden syndrome, an autosomal-dominant multiple hamartoma-neoplasia syndrome caused by germline mutations in the tumor suppressor gene (PTEN) on chromosome number 10. [18] - Hemangioblastoma
Hemangioblastomas are benign tumors that arise in the cerebellum, brainstem, and/or spinal cord/canal, that occur sporadically (often solitary) or are associated with von Hippel–Lindau disease (usually multiple lesions). [18] - Medulloblastoma (MB)
Medulloblastomas are malignant embryonal tumors of the cerebellum. Although prevalent across the entire age spectrum, they are extremely rare in adults (< 1% of adult CNS tumors). In children aged 0–14 years, MB is the most common embryonal brain tumor with an age-adjusted incidence rate of 0.49 per 100,000. There are four molecular subgroups of MB – wing-less (WNT), sonic hedgehog (SHH), group 3, and group 4 – with distinct clinical features, cells of origin, pathogenesis, and outcome. WNT-MBs are believed to originate from dorsal brainstem progenitors of the lower rhombic lip and arise at the level of the cerebellopontine angle and cerebellar peduncle. They account for 10% of MBs and display WNT pathway activation, due to mutations in (CTNNB1) gene encoding beta-catenin. With a low propensity to metastasize and a good response to different treatment regimens (overall survival of more than 90%), WNT tumors have the best prognosis of all four subgroups. [18]
Conclusion
The cerebellum is a part of the brain that is responsible for the coordination of voluntary motor movement, balance and equilibrium, and muscle tone. It is located at the back of the brain, underlying the occipital and temporal lobes of the cerebral cortex. Damage or degeneration to cerebellar structures can lead to serious conditions and syndromes such as ASD & ataxia among others.