Article Topic: Normal Pressure Hydrocephalus (NPH)
Author: Tasneem Khallad
Editor: Philip Sweidan, Ethar Hazaimeh
Keywords: iNPH, sNPH, Hakim’s triad, CSF shunt.
Overview
Normal-pressure hydrocephalus (NPH) is a neurological disorder first described in 1965 and considered to be the first treatable type of dementia ever known. (1) It is a potentially reversible syndrome that presents with dilated cerebral ventricles in combination with a triad of symptoms; urinary incontinence, gait impairment, and dementia. Diagnosis is based on medical history, neurological examination, and brain imaging. CSF ventriculoperitoneal shunting is the most commonly used treatment modality for NPH. (2)
Introduction
Hydrocephalus refers to the distension of the cerebral ventricles due to abnormal accumulation of cerebrospinal fluid (CSF) and is said to be of two types: communicating or obstructive. If the accumulation is because of the increase in intracranial CSF content, this is a communicating or non-obstructive hydrocephalus, where the flow of CSF from the ventricular system to the lumbar subarachnoid space is not blocked and communication between the ventricles is still maintained. On the other hand, it is considered noncommunicating or obstructive when the accumulation of CSF is due to a blockage in the pathway of CSF flow. (1) The subject of this article will be the communicating type of hydrocephalus.
More than 50 years ago, Raymond Adams and Salomón Hakim described 3 cases of adults who had chronic symptomatic hydrocephalus with normal CSF pressure and a triad of symptoms, commonly known as the Hakim triad, including urinary incontinence, dementia, and gait disturbances. (3) They also reported remarkable restoration of neurologic function after CSF shunting. (1)
Pathophysiology
NPH is divided into two different entities: idiopathic (iNPH) and secondary (sNPH). iNPH accounts for 50% of total cases, occurring most commonly in older adults due to unknown causes. In contrast, sNPH occurs in any age group as a result of an underlying disease, such as meningitis, subarachnoid hemorrhage, or cranial trauma. (4,5) A common feature of both is that men and women are equally affected. (5) Also, the two carry a similar prognosis. (6–9) Whereas weeks to months are required for sNPH to develop after the onset of the initial incident, the preclinical stage for iNPH is gradual over years. (10) Although it is called “normal pressure” hydrocephalus, the CSF pressure is initially elevated, and once that raised pressure had caused ventricular distension, it drops again to normal levels while ventriculomegaly is maintained. (1)
Idiopathic NPH
It is not yet understood what mechanisms can lead to the increase in CSF volume in iNPH. The earliest theories referred to the increase in CSF volume to be a result of a decrease in CSF absorption and therefore, increased ventricular volume as a compensatory mechanism to maintain normal CSF flow. (11)
Many studies have shown an association between vascular risk factors and iNPH pathophysiology, where patients with iNPH had a history of hypertension, diabetes mellitus (DM), or white matter lesions (WML). (12–15)
WML is thought to cause iNPH through microvascular disturbances in the white matter. Remarkably, patients with iNPH who were found to be having reduced blood flow in the periventricular white matter have shown improved blood flow after CSF removal. (16–19) Also, postoperative reduction of WML has improved clinical symptoms in iNPH. (20,21)
However, DM and hypertension probably increase the risk of iNPH through different mechanisms than that of WML as they increase the risk of iNPH independent of WML. One of the assumptions said that hypertension could cause iNPH through a direct mechanical effect on ventricular size. (1)
Secondary NPH
In order to consider NPH as secondary, a well-established event should precede the onset of symptoms of NPH. (22) Subarachnoid hemorrhage (SAH) is considered the leading cause of sNPH, accounting for 45.6% of the total cases. 7-37% of those patients develop chronic hydrocephalus. (23) The mechanism by which SAH causes sNPH is through fibrosis and adhesion in the subarachnoid space and arachnoid granulations. (22) 6.2% of sNPH cases occur secondary to intracranial malignancies as tumors release proteinaceous products as well as cellular components into the CSF, increasing its viscosity and resulting in impaired reabsorption at the level of the arachnoid granulations. (24) NPH can also occur post-traumatically when there is an impairment in CSF dynamics (i.e., absorption, or flow). Post-traumatic NPH has a wide variation in incidence, ranging from 1% to 29%. (10) However, patients with sNPH may show other atypical symptoms like seizures, motor and sensory deficits, and altered consciousness. This is explained by severe neurological deficits caused by the primary disease masking the classical NPH manifestations. (25)
Clinical Features
NPH patients present with three cardinal symptoms that are not always seen in all patients and differ between presenting cases depending on their onset, severity, and progression. (26) These include:
- Impairments of Gait and Balance
They are typically the earliest clinical manifestations to be noticed and considered the most responsive to CSF shunting. (27) Initially, patients will have difficulty walking on stairs and slopes and will struggle with transitional movements (standing to sitting or sitting to standing). (26) As the disease progresses, the gait becomes short-stepped and broad-based with reduced speed and step heights, therefore described as “magnetic” or “glued to the floor”. (28) Slow movement of upper extremities is also common. (29)
- Urinary Incontinence
Bladder function will be disturbed as a result of detrusor overactivity. This disturbance presents in a gradual course, starting with increased urinary frequency, later, urge continence, and finally, permanent incontinence. (4,30) And It improves with CSF shunting in about 80% of patients if performed early. If performed in advanced stages, urinary incontinence improves in only 50-60% of cases. (31–33)
- Cognitive Impairment and Dementia
The earliest cognitive symptoms in NPH patients are impaired attention and concentration, psychomotor slowing, executive and visuospatial dysfunction, and short-term memory impairment occurring mainly due to subcortical frontal dysfunction. (34) Later, bradyphrenia, reduced speech production, apathy, reduced drive, and indifference can occur. (8,9,35,36) Of note, patients with NPH do not show psychiatric abnormalities, therefore, signs of mood, personality, and behavior changes may exclude NPH from diagnosis. (37) CSF shunting improves cognitive decline but this is less likely to happen if the patient has Alzheimer’s or vascular dementia concomitantly. (37)
Differential Diagnosis
When Hakim’s triad is incomplete or its manifestations are atypical, the differential diagnosis may be steered toward another disease making the process of diagnosis more difficult. Moreover, the complete triad can be caused by other diseases than NPH. As a result, complementary tests, including neuroimaging and CSF tap test should be done to aid in the processes of NPH diagnosis and management.
Table 1: Differential diagnosis of NPH (26,38).
| Vascular Dementia
Stroke Multi-infarct state Cerebrovascular disease Binswanger’s disease Leukoencephalopathy Vertebrobasilar insufficiency Cerebral autosomal dominant arteriopathy Subcortical infarct |
| Neurodegenerative Disorder
Alzheimer’s disease Parkinson’s disease Huntington’s disease Lewy body disease Frontotemporal dementia Multisystem atrophy Amyotrophic lateral sclerosis Spongiform encephalopathy Progressive supranuclear palsy Corticobasal degeneration |
| Infectious Disease
Lyme Syphilis Human immunodeficiency virus Progressive multifocal leukoencephalopathy |
| Other hydrocephalus disorders
Arrested hydrocephalus Aqueductal stenosis Long-standing overt ventriculomegaly syndrome Noncommunicating – obstructive – hydrocephalus |
| Urological diseases
Urinary tract infection Benign prostatic hyperplasia Prostate or bladder cancer |
| Others
Depression Epilepsy Collagen vascular disorder Traumatic brain injury Spinal stenosis Spinal cord tumor Brain tumor Organ failures Hypothyroidism Peripheral neuropathy B12 deficiency Chiari malformation Carcinomatous meningitis Wernicke’s encephalopathy Chronic subdural hematoma |
Diagnosis
Diagnosis is done by the evaluation of clinical manifestations, neuroimaging, and CSF dynamics parameters combined. In the past, the presence of the complete triad was obligatory for the diagnosis and treatment of NPH, but nowadays the presence of two out of the three symptoms is enough to diagnose and start the treatment of NPH. (1,3,39) or even one symptom is enough. (9,35) This is explained by the fact that the presence of the full triad represents an advanced stage and thus worsened prognosis if remained untreated. (9)
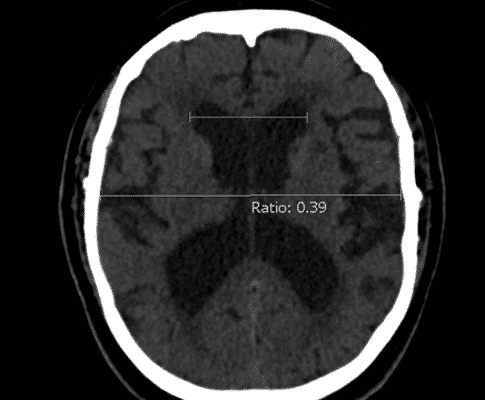
Ventriculomegaly is considered the only definitive diagnostic finding for NPH,(40) which is measured by Evans’ index (the ratio between the maximal width of the frontal horns and the widest brain diameter). (Figure 1) Some findings tend to decrease the probability of NPH including cortical deficits, asymmetrical manifestations, progressive dementia in the absence of gait disturbances, and lack of progression of symptoms. (37)

Figure 1: Evan’s index. Case courtesy of Dr Yuranga Weerakkody (41).
International and Japanese guidelines were published on NPH for its diagnosis.
The International Guidelines include the following key imaging features (42):
- Ventricular enlargement with Evans index that is more than 0.3.
- No obstruction to CSF flow microscopically.
- At least one of these supportive features:
– Enlarged temporal horns of the lateral ventricles, are not entirely attributable to hippocampus atrophy.
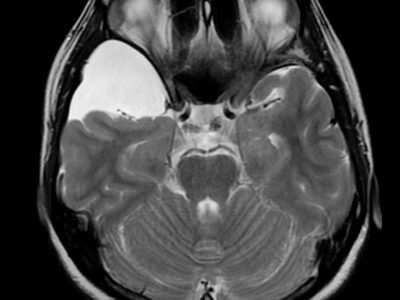
– Callosal angle of 40º or greater (Figure 2).
– Periventricular signal changes on CT and MRI, not due to microvascular ischemic changes or demyelination, indicating altered brain water content.
– Flow void on MRI in the Sylvian aqueduct or 4th
According to The Japanese Guidelines, periventricular changes are not necessary, and the following key imaging features are required for NPH diagnosis (43):
- Enlarged Sylvian fissures and basal cistern.
- Narrowing of the sulci and subarachnoid spaces over the high convexity and midline surface of the brain.
In NPH, the volume of CSF could also increase in the subarachnoid space along with the ventricular spaces, accumulating in the major fissures of the brain, and thus displacing the surrounding brain tissue. This leads to compressed adjacent sulci resulting in what is known as disproportionately enlarged subarachnoid space hydrocephalus (DESH), which is included in the Japanese guideline for NPH diagnosis. (Figure 3) Tight high convexity and dilated Sylvian fissures with ventriculomegaly are characteristics of DESH. (40) However, DESH is proven to be associated with a good response to CSF shunt. (39)
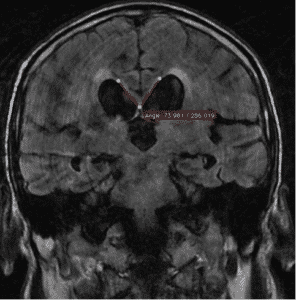
Figure 2: Callosal angle. Case courtesy of Dr. Andrei Tsoriev (45).
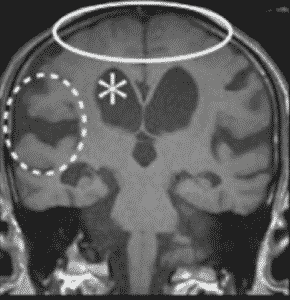
Figure 3: Disproportionately enlarged subarachnoid space hydrocephalus (DESH) showing ventriculomegaly (*), enlarged Sylvian fissure (dotted circle), and high convexity tightness (solid circle) (46).
Diagnostic Techniques
Complementary tests should be used along with clinical findings assessment to reach a better diagnosis and management. Diagnostic techniques include gait and urinary symptoms assessment, neurological examination, CSF dynamics assessment, laboratory tests, and imaging modalities.
Imaging Modalities
Neuroimaging is routinely used as the first diagnostic tool whenever NPH is suspected, looking for ventricular enlargement that is not due to atrophy, CSF collection outside the ventricles, and documenting the absence of macroscopic CSF flow obstruction, thus ruling out other pathologies. (9) Magnetic resonance imaging (MRI) is the method of choice but computed tomography (CT) could be an acceptable alternative. (9)
Other neuroimaging methods can be used, but are not considered as part of the routine diagnostic procedures, including positron emission tomography (PET) and single-photon emission computed tomography (SPECT) which show reduced cerebral blood flow and metabolism. (47) Also, diffusion tensor imaging (DTI) and resting-state functional MRI, which are newer MRI techniques, allow the detection of NPH biomarkers. (48,49)
Shunt Effectiveness Evaluation
The international guidelines recommend testing CSF dynamics to evaluate whether improvement after shunting is possible. (50,51) The idea of these tests is to temporarily simulate the effect of shunting by draining a certain amount of CSF and examining the patient’s gait and cognition before and after. If the patient has NPH, a significant response to CSF drainage happens and shunting is predicted to be helpful. CSF tap test (CSF-TT) is done by draining 40-50 ml of lumbar CSF. False-negative results are suspected due to its low sensitivity, which is about 26-61%. In these cases, surgery is not excluded, but a repeated CSF-TT (RTT) is performed by removing 30-40 ml CSF for three consecutive days.
Another alternative procedure is called external lumbar drainage (ELD) in which a minimum of 150 ml of CSF is drained daily for 3 to 5 days. ELD is superior to the others and is considered the most effective test for identifying shunt-responsive cases due to its high sensitivity (50-100%) and high positive predictive value (80-100%). However, ELD requires hospitalization and complications are liable to develop such as nerve root inflammation, subdural hematoma, and meningitis. The third one is the most important and is seen in about 2-3% of patients. (15,42)
Shunt Patency Evaluation
Lumbar infusion test and radionuclide cisternography are done to evaluate CSF shunt malfunction.(52,53) Radionuclide cisternography is considered a simple, effective, and low-radiation dose method in which a small volume radioisotope is injected into the shunt reservoir, then images are obtained every minute for 20 minutes to create a time-activity curve. (54) Lumbar infusion test depends on measuring the CSF outflow resistance (Rout), a measure of the resistance to CSF resorption, as an indicator for shunt function. This is done by infusing ringer lactate through one spinal needle and recording CSF pressure through a second spinal needle at the same time.(55) If the shunt is obstructed, Rout value will be the same as before the surgery but if it is patent, the values after will be lower than before. (56)
Treatment
The first-line treatment for NPH is CSF shunting. It can be done by implanting a ventriculoperitoneal, ventriculopleural, or ventriculoatrial shunt where CSF is drained from the lateral ventricles to different body areas. (2) The standard procedure is done using a ventriculoperitoneal shunt. This intervention leads to improvement in symptoms, being more significant in sNPH patients; up to 70% compared to 50% seen in iNPH individuals. (57) The best shunting results are achieved when the CSF tap test is positive along with MRI and CT showing the key features of NPH.
Conclusion
NPH faces a serious problem of underdiagnoses; up to 80% of patients remain unrecognized and thus, inappropriately treated. (37) Hence, it should always be taken into consideration as a differential diagnosis whenever its characteristics are present and treated as early as possible when diagnosed.