ARACHNOID CYSTS
Author : Soliman Mohamed Abdelmonem Soliman
Editor: Menna Mohamed Marwan.
Scientific Editor :- Dr. Adam M. Abdallah
keywords: (Arachnoid cyst, Sylvian Arachnoid Cysts, Intraventricular Arachnoid Cysts, minimally invasive cyst fenestration)
Overview :
Arachnoid cysts are fluid-filled duplications or detachments of the arachnoid membrane in concentrations similar to but not equal to cerebrospinal fluid. Arachnoid cysts are not a true neurodegenerative disorder, but an underlying defect in the structure of the arachnoid layer that may be congenital. They may occur sporadically or in combination with other malformations or illnesses. Arachnoid cysts can be detected during childhood. However, they can grow de novo, enlarge, or decrease in size. They can be diagnosed by screening ultrasound during fetal life or detected during childhood or adulthood. Many arachnoid cysts are asymptomatic.
Treatment strategies are controversially discussed. If they are diagnosed incidentally or if they are associated with very mild symptoms, conservative management with follow-up imaging may be preferred. If they grow larger, they can cause headaches, seizures, or other neurological symptoms and require neurosurgical treatment. This chapter discusses aspects of the pathogenesis, clinical symptoms, indications for neurosurgical treatment, and treatment options.(1,2,3)
Pathogenesis :
True AC is a benign lesion and is thought to be developmental, or at least based on a congenital error in the structure of arachnids. It should be noted that
arachnoid septa of varying strength are woven through the subarachnoid space, especially at the base of the skull and in the region of the Sylvian fissure. Physiologically, many inner arachnoid membranes can form the subarachnoid cistern. (4)
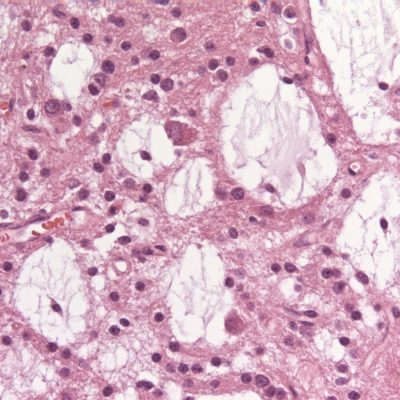
Microstructurally, the wall of AC is similar to the normal arachnoid. Miyagami and Tsubokawa analyzed the surgically resected atrioventricular wall using light and electron microscopy and found that the inner surface of the atrioventricular wall consists of one or more layers of arachnoid cells with thin cylindrical projections and large intercellular spaces. (5) Rengachary and Watanabe observed structural differences from the normal arachnoid: ( I ) the splitting of the arachnoid membrane at the margin of the cyst, (2) a thicker layer of collagen in the cyst wall, (3) the absence of traversing trabecular processes within the cyst and (4) the presence of hyperplastic arachnoid cells in the cyst wall, which participate in collagen synthesis. (6)
The pathogenesis of AC is still not fully understood. Meningeal tissue is of mesectodermal origin and derives from the neural crest. AC, as well as meningiomas, may be located intraventricularly, which indicates that mesectodermal tissue may be dislocated into the neural tube during the folding of the latter, which begins around the 20th embryonic day. A network of mesenchymal cells develops between the brain and the epidermis and eventually differentiates into a dense outer layer and a reticular inner layer to form the meninges. The inner layer further differentiates to form the dura mater and the leptomeninges. (7) The frequent occurrence of these phenomena in childhood, histological features without changes after inflammation or hemorrhage, and their occurrence with genetic disorders strongly suggest the nature of development. However, this view has recently been challenged. Temporal lobe
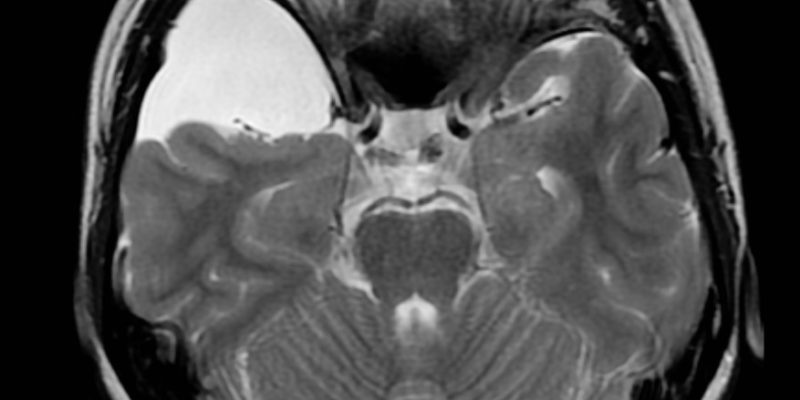
and Sylvian localization predominate in children and adults,(8,9) whereas in prenatal analyzes the majority of AC are interhemispherically located and temporal AC is extremely rare (10) (Table 1). This observation casts some doubt on the hypothesis that AC formation is strictly congenital. As they may develop de novo in adults, may grow over time, furthermore, the cyst size on the first diagnosis is larger with increasing age. (11,12)
Mechanisms of cyst-growth
Berle and colleagues systematically analyzed intracystic fluid and found different levels of phosphate, protein, ferritin, and lactate dehydrogenase in comparison to normal cerebrospinal fluid. A possible mechanism of cyst growth should therefore be consistent with these findings. Many mechanisms have been proposed. Possible mechanisms could be active secretion by the cells of the cystic wall, expansion along an osmotic pressure gradient, or a one-way valve mechanism. (13)
-
Active Fluid Secretion
In an ultrastructural and cytochemical study, the resected walls of AC were analyzed. They observed similarities between the neurothelial lining of arachnoid granulations. On the luminal surface, the structure’s cyst wall is densely covered with microvilli, thus, suggesting active secretion. Enzyme cytochemistry demonstrated Na-K-ATPase at the Luminal plasma membrane and alkaline phosphatase at the opposite membrane. This architecture could indicate fluid transport towards the lumen. Different consistency compared to Cerebrospinal fluid may be explained by this hypothesis. (14)
-
Osmotic Gradient
The hypothesis that AC evolves along an osmotic gradient has been suggested due to the small difference in substance concentrations from the CSF. A thin wall with loose connective tissue may allow the permeation of water. However, the formation of an osmotic gradient must be activated. In secondary cysts, hemorrhage with hemolysis products or an inflammatory response can act as an osmotic activator. In primary AC, there is no evidence that these mechanisms exist. (15)
-
Valve Mechanism
Small and medium-sized cysts remain the same size and readily communicate with the normal subarachnoid space.(12,16) In larger cysts or growing cysts, a valve mechanism may develop with unidirectional fluid transport. In the case of positive intradermal/ ectodermal pressure, the cyst may enlarge. Valvular folds of the cyst wall or endometrium have been observed during laparoscopic or open procedures. (17,18)
Clinical picture
Clinical symptoms are determined by the location of the cyst. AC can occur anywhere in the skull and spine where the arachnoid is present. Typical symptoms of AC are described below.
-
Temporal and Sylvian Arachnoid Cysts
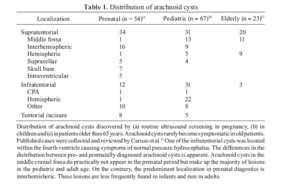
The middle cranial fossa is the most common location for AC. In a large series of pediatric and adult patients, about two-thirds of them suffer from AC in the middle cranial fossa or Sylvian fissure. Bilateral temporal AC is rarely seen, and these patients may suffer from glutaric aciduria. (19) Most commonly, these patients present with headaches and seizures, and contralateral movement disorders. Developmental delays and delays are more common in patients with the apex of the sphenoid bone of AC. (20,21) The link between temporary AC and psychiatric and neurobehavioral disorders is still under debate. Various cases of AC-related psychiatric disorders have been reported. These include attention deficit hyperactivity disorder, depression, hallucinations, dementia, schizophrenia, anorexia nervosa, insomnia, and other psychotic disorders. A systematic preoperative and postoperative assessment of cognitive function was published by Wester et al. They found that many patients with left temporal lobe AC had significant defects in various cognitive functions such as language perception, memory function, complex language tasks, visuospatial function, and visual attention.(22,23,24)
-
Intraventricular Arachnoid Cysts
Intraventricular AC is rare. They were found in the lateral ventricles, third ventricle, and fourth ventricle. These cysts become clinically prominent through the development of acute or chronic hydrocephalus as the size of the cyst increases. Symptoms due to compression of surrounding structures have also been reported. For example, AC in the 4th ventricle can cause symptoms such as cerebellar dysfunction or depression. (25)
-
Cerebellopontine Angle and Retrocerebellar Arachnoid Cysts
Infratentorial location forms about 10% of all AC. The main site of these is located in the cerebellopontine angle or retrocerebellar. Symptoms of retrocerebellar cysts include hydrocephalus and compression of cerebellar or brainstem structures. Patients predominantly present with headaches or gait disturbances. Cerebellopontine angle AC may obstruct the CSF circulation in the posterior fossa and cause obstructive hydrocephalus. Cerebellopontine angle structures may be directly compressed or affected in terms of neurovascular compression. Cranial nerve symptoms may include hearing loss, trigeminal nerve neuralgia, hemifacial muscle spasm, diplopia, hoarseness, and dysphagia. (26-32)
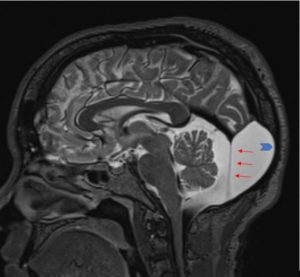
-
Supra- and Intrasellar Arachnoid Cysts
Intra- and suprasellar AC occur near the optic nerve, pituitary and pituitary stalks, hypothalamus, and mesencephalon. In the case of large suprasellar extension, they can interfere with the physiological outflow of cerebrospinal fluid at the level of the third ventricle or the foramen of Monroe, thus causing obstructive hydrocephalus. Visual impairment and endocrine disorders can be caused by compression of the optic nerve and compression of the pituitary gland or pituitary stalk. Compression of the hypothalamic structures can cause developmental disorders such as precocious puberty. Bobblehead puppet syndrome can be caused by compression of the third ventricle or thalamic nucleus. This is a clinical feature that strongly suggests saddle AC. However, this typical feature is found only in a small number of these patients. (33)
-
Spinal Arachnoid Cysts
Like cranial AC, spinal cord AC usually causes symptoms due to compression of the spinal cord and radiculopathy Myelopathy symptoms such as ataxia, recurrence of primitive reflexes, and progressive paralysis can be caused by compression of the cord. symptoms of radiculopathy due to termination of radiculopathy may include pain, sensory deficits, or weakness of the effector’s muscles. (38)

Diagnostic procedures
In many patients with intracranial and spinal AC, it is difficult to assess the
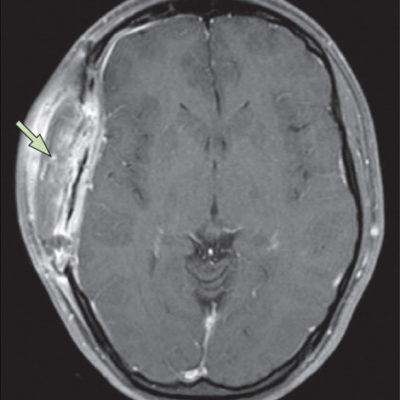
indication for surgical treatment. The success of the treatment strategy is based on the correlation between lesions and clinical manifestations. A detailed interview with the patient or the patient’s parents and a thorough neurological examination is essential to correlate signs and symptoms with radiation findings. This particular probably counts random cysts. (38) Laboratory investigations for screening of glutaric aciduria must be recommended in patients with bilateral AC. In fetuses with AC detected by routine ultrasound screening, karyotype evaluation, and fetal MRI should be performed to rule out a possible association with other malformations. Electroencephalography is essential in patients with supratentorial AC to determine the necessity for antiepileptic medication. In spinal AC cord compression may be further evaluated by somatosensory and motor evoked potentials. Magnetic resonance imaging (MRI) is the mainstay of radiological diagnostics. Due to their worse imaging quality and because of X-ray exposure, computed tomography scan (CTS) is, especially in children, not acceptable as a diagnostic tool. Modem phase-contrast MRI and functional MRI are not only capable of demonstrating structural abnormalities but also able to depict CSF-flow patterns and functional organization of the brain respectively. Cisternography with contrast filling of the subarachnoid space followed by CTS, sometimes at more than one time-point to assess the time-course of contrast filling of CSF-compartments, is reserved for very few cases of diagnostic uncertainty. (38)
Treatment
There are several options for treating AC. Resection of the cyst wall requires open surgery. Cyst fenestration in the subarachnoid cistern can be done endoscopically or by open surgery microsurgical techniques. Various shunt techniques have been reported, including cystic peritoneal and subdural shunts. The optimal surgical strategy is still under debate. Which type of treatment is selected individually depends on a variety of factors. Patient symptoms, cyst location and shape, the extent of the cystic septum, proximity to relevant nerve and vascular structures, and experience of the surgeon’s various techniques. Observations for patients with no associated symptoms and no associated neurological defects observation are recommended. (38) If there is an indication for treatment, endoscopic fenestration can be recommended as the treatment of the first choice for many AC, in particular, if there are no relevant intracystic septations. If suprasellar arachnoid cysts are present, promising results have been reported. Ventriculocystostomy and ventriculocystocistemostomy appear to be the most successful treatment in the long term. (38) Temporal and temporobasal AC appear to be more difficult to be treated by endoscopic strategies. (34) If there are intracystic septations or multiple fine neural structures like in the cerebellopontine angle, the recurrence rate might be higher if treatment only consists of cyst-fenestration. Complete or near-complete excision of the cyst wall or adherences supplies the most successful treatment at these locations. (35) For small or medium-sized temporal cysts, excision or fenestration shows a good chance of complete disappearance of the cyst and complete re-expansion of the adjacent temporal lobe. For large cysts, only incomplete expansion of the adjacent brain has been demonstrated. Additional shunt placement may be needed. Shunting procedures are only a little invasive, and supply good control of the cyst volumes by pressure-controlled drainage, but may have a relatively high failure rate. (37) Fenestration or excision of the cyst may bear a higher operative risk but leaves the patient shunt independent. A long-term success rate of 76-90% has been reported for these ways of treatment.(34,36,37)
References :
- Bright R. Serous cysts in the arachnoid diseases of the brain and nervous system, part I: reports of medical cases selected with a view of illustrating the symptoms and cure of diseases by a reference to morbid anatomy. 1831; 2:437439.
- Cunningham DJ. A Large sub-arachnoid cyst involving the greater part of the parietal lobe of the brain. Anat Physiol 1879; 13:508-517.
- Robinson RG. The temporal lobe agenesis syndrome. Brain 1964; 87:87-106.
- Inoue K, Seker A, Osawa S, et al. Micrbsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery 2009; 65:644-664.
- Miyagami M, Tsubokawa T. Histological and ultrastructural findings of benign intracranial cysts. Noshuyo Byori 1993; 10:151-160.
- Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol 1981; 40:61-83.
- Angelov DN, Vasilev V A. Morphogenesis of rat cranial meninges. A light- and electron-microscopic study. Cell Tissue Res 1989; 257:207-216.
- Pascual-Castroviejo I, Roche MC, Martinez BA et al. Primary intracranial arachnoidal cysts. A study of 67 childhood cases. Childs Nerv Syst 1991; 7:257-263.
- CarusoR, Salvati M, Cervoni L. Primary intracranial arachnoidcystintheelderly. NeurosurgRev 1994; 17:195-198.
- Pierre-Kahn A, Hanlo P, Sonigo P et al. The contribution of prenatal diagnosis to the understanding of malformative intracranial cysts: state of the art. Childs Nerv Syst 2000; 16:619-626.
- Struck AF, Murphy MJ, Iskandar BJ. Spontaneous development of a de novo suprasellar arachnoid cyst. case report. J Neurosurg 2006; 104:426-428.
- Becker T, Wagner M, Hofmann E et al. Do arachnoid cysts grow? A retrospective CT volumetric study. Neuroradiology 1991; 33:341-345.
- Holmes LB, Redline RW, Brown DL, et al. Absence, “hypoplasia of tibia, polydactyly, retrocerebellar arachnoid cyst, and other anomalies: an autosomal recessive disorder. J Med Genet 1995; 32:896-900.
- Ito J, Yamazaki Y, Honda H, et al. Angiographic appearance of a huge retrocerebellar arachnoid cyst in a Neuroradiology 1977; 13:1 15-119.
- Cagnoni G, Fonda C, Pancani S et al. Intracranial arachnoid cyst in pediatric age. Pediatr Med Chir 1996; 18:85-90.
- Galassi E, Tognetti F, Gaist G, et al. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol 1982; 17:363-369.
- Alehan FK, Gurakan B, Agildere M. Familial arachnoid cysts in association with autosomal dominant polycystic kidney disease. Pediatrics 2002; 1 10:13.
- Arriola G, de Castro P, Verdu A. Familial arachnoid cysts. Pediatr Neurol 2005; 33:146 148.
- Hald JK, Nakstad PH, Skjeldal OH, et al. Bilateral arachnoid cysts of the temporal fossa in four children with glutaric aciduria type 1. AJNR Am J Neuroradiol 1991; 12:407-409.
- Koch CA, Voth D, KraemerG et al. Arachnoid cysts: does surgery improve epileptic seizures and headaches. NeurosurgRev 1995; 18:173-181.
- Arai H, Sato K, Wachi A, et al. Arachnoid cysts of the middle cranial fossa: experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery 1996; 39:1108-11 12.
- Smith RA, Smith WA. Arachnoid cysts of the middle cranial fossa. Surg Neurol 1976; 5:246-252.
- Brugger G. Cerebral arachnoid cysts in children with local bulging of the skull. Wien Klin Wochenschr 1976; 88:764-769.
- Wester K. Intracranial arachnoid cysts—do they Impair mental functions? J Neurol 2008; 255:1113-1120.
- Szucs A, Varady P, Pestality P et al. Occlusive hydrocephalus caused by a fourth ventricle arachnoid cyst. Ideology Sz 2008; 61:54-58.
- Cartwright, Ei senberg MB, Page LK. Posterior fossa arachnoid cyst presenting with an isolated twelfth nerve paresis. Case report and review of the literature. Clin Neurol Neurosurg 1991 ; 93:69-72.
- Mastronardi L, Taniguchi R, Caroli M, et al. Cerebellommtine angle arachnoid cyst: a case of hemifacial spasm caused by an organic lesion other than neurovax-ular compression case report. Neurosurgery 2009; 65:1205.
- Ashker L, Weinstein JM, Dias M, et al. Arachnoid cyst causing third cranial nerve palsy manifesting as isolated internal ophthalmoplegia and iris cholinergic supersensitivity. J Neuroophthalmol 2008; 28: 192-197.
- Hayden MG, Tomabene SV, Nguyen A, et al. Cerebellopontine angle cyst compressing the vagus nerve: case report. Neurosurgery 2007; 60: 1150.
- Chao TR. Middle cranial fossa arachnoid cysts causing sensorineural hearing loss. Eur Arch Otorhinoluyngol 2005; 262:925-927.
- Pirotte B, Morelli D, Alessi G, et al. Facial nerve palsy in posterior fossa arachnoid cysts: report of two cases. Childs New Syst 2005; 21:587-590.
- Achill I V, Danesi G, Cavemi L, et al. Petrous a}EX arachnoid cyst: a case report and review of the literature. Acta Otorhinolaongol Ital 2005; 25:296-300.
- Starzyk J, Kwiatkowski S, Urbanowicz W et al. Suprasellar arachnoidal cyst as a cause of precocious puberty—report of three patients and literature overview. J Pediatr Endocrinol Metab 2003; 16:447-455.
- Oertel JM, Wagner W, MondorfY, et al. Endoscopic treatment of arachnoid cysts: a detailed account of surgical techniques and results. Neurosurgery 2010; 67:824-836.
- Samii M, Carvalho GA, Schuhmann MU et al. Arachnoid cysts of the posterior fossa. Surg Neurol 1999; 51:376-382.
- Galassi E, Piazza G, Gaist G, et al. Arachnoid cysts of the middle cranial fossa: a clinical and radiological study of 25 cases treated surgically. Surg Neurol 1980; 14:211-219.
- Raffel C, McComb JG. To shunt or to fenestrate: which is the best surgical treatment for arachnoid cysts in pediatric patients? Neurosurgery 1988; 23:338-342.
- Westermaier T, Schweitzer T, Ernestus RI. Arachnoid cysts. Adv Exp Med Biol. 2012;724:37-50. doi: 10.1007/978-1-4614-0653-2_3. PMID: 22411232.