Topic: Acquired Metabolic and Toxic Disturbances.
Author: Taqwa Haweeleh.
Editor: Yazan S. Al-Zamer
Keywords: hypoglycemia, brain damage, hyperglycemia, diabetes mellitus, diabetes, hyperglycemic hyperosmolar syndrome, HHS, ketoacidosis, diabetic ketoacidosis, hepatic encephalopathy.
Hypoglycemia
Insulin secretion from pancreatic β cells is regulated depending on the body’s glucose levels, insulin acts as a glucose sensor, the corollary being that insulin secretion is regulated to prevent hyperglycemia or hypoglycemia. Diabetic patients treated with insulin therapy are the most susceptible group of patients to hypoglycemia, whether it is type 1 or type 2, where type 1 occurs due to loss of the insulin-secreting β cells of the pancreas and type 2 occurs because of reduced response to insulin. Hypoglycemia is a medical condition in which the body’s glucose falls below normal levels, and hyperglycemia is the opposite. Hypoglycemia can arise due to multiple causes other than diabetes such as starvation, kidney failure, liver disease, some tumors, severe infections, inborn errors of metabolism, and certain drugs including alcohol. Prolonged hypoglycemia is suspected to cause brain damage in the cortex and regions within the hippocampus depending on its severity. Rarely, extreme hypoglycemia causes brain death; animal studies revealed that neuronal death happens when blood glucose levels fall below 1 mM for an extended period, correlated with an isoelectric ‘‘flat-line’’ EEG. 2. 2
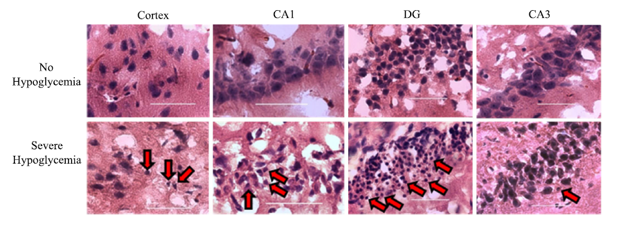
This figure shows the results of a study done on rats, the rats that did not undergo hypoglycemia in comparison to rats who underwent severe hypoglycemia. The rats that underwent severe hypoglycemia developed brain damage in the cortex and certain regions in the hippocampus [CA1 and dentate gyrus (DG)], the cells’ morphology revealed brain cells characterized by condensed shrunken morphology and pyknotic nuclei (red arrows) with eosinophilic staining. 3
This figure shows the results of a study done on rats, the rats that did not undergo hypoglycemia in comparison to rats who underwent severe hypoglycemia. The rats that underwent severe hypoglycemia developed brain damage in the cortex and certain regions in the hippocampus [CA1 and dentate gyrus (DG)], the cells’ morphology revealed brain cells characterized by condensed shrunken morphology and pyknotic nuclei (red arrows) with eosinophilic staining. 3
Glucose is considered the most vital energy substrate in brain metabolism; the brain functions are consequently at risk when it is deprived of adequate glucose supplies. The brain is unable to synthesize glucose or store considerable amounts of glycogen in astrocytes, thus requiring a virtually continuous supply of glucose from the circulation. In a healthy individual, counter-regulatory mechanisms protect the brain from the effects of hypoglycemia if a sudden fall in plasma glucose occurs, these autonomic counter-regulatory responses include the inhibition of insulin secretion and the sequential release of glucagon, epinephrine, norepinephrine, cortisol (ACTH), and growth hormone. However, patients who experience recurrent bouts of hypoglycemia may subsequently exhaust their counter-regulatory mechanisms rendering them impaired, which makes detecting hypoglycemia by the body’s autonomic system harder and causes states of hypoglycemia unawareness thereby endangering the brain. Brain glycogen stores can be used as a source of glucose under extreme hypoglycemia. However, one study concluded that glycogenolysis can account for the majority of the glucose deficit through the slow rates of glycogen depletion and its inability to provide adequate energy supplies in cases of prolonged hypoglycemia. 4 5
Mild hypoglycemia occurring in both healthy and diabetic patients can affect cognitive functions, cognitive impairment is associated with vascular changes in the brain causing symptoms such as reduced concentration, blunted short-term memory, and weakened verbal skills. Studies conducted on patients treated with insulin or other hypoglycemic medications revealed that neurons within the hippocampus are the most affected by hypoglycemia, followed by neurons within the basal ganglia and thalamus. However, neurons within the brain stem, cerebellum, and spinal cord appeared to be more resistant to hypoglycemia. cerebral autopsy preparations from hypoglycemic cases also exhibited traces of myelin damage and glial changes; glial changes due to hypoglycemia include swelling and proliferation of astrocytes and oligodendrocytes in regions of neuronal damage. 6 7
Hypoglycemia is the most prevalent metabolic problem in newborns, and it occurs in about 19% of infants overall and reaches up to 51% in neonates at high risk for hypoglycemia. Neonatal hypoglycemia can be either asymptomatic or mildly symptomatic, yet its symptoms are rather nonspecific and subtle, and vary wildly between patients. Infants with hypoglycemia are at increased risk for brain injury and so it is crucial to ensure they reach a euglycemic state. There are numerous risk factors for neonatal hypoglycemia and can be related to the mother or the baby itself, one of which is hyperinsulinemia caused by maternal gestational diabetes or type 2 diabetes mellitus (DM), IUGR, post maturity, placental insufficiency, birth asphyxia. Another risk factor is increased glucose utilization in sick or stressed infants due to perinatal hypoxia or ischemia. The exact blood glucose number for determining neonatal hypoglycemia is still unclear, furthermore, an ideal treatment strategy is yet to be established. 8
Hyperglycemia
The brain was signified to be an important target of diabetic complications by previous research. Diabetes, whether it be type 1 or type 2, is a chronic, metabolic disease characterized by hyperglycemia which over time will cause serious damage to the blood vessels in the eyes, kidneys, nerves, and heart. Furthermore, chronic hyperglycemia in diabetic patients may lead to the disruption of the brain’s topological integration, thereby causing mental slowing and cognitive impairment. To ascertain the correlation between white matter microstructural alteration and glycemic control, voxel-wise TBSS on DTI images was performed to determine if the altered brain networks in diabetic participants are due to microstructural changes in the local regions of white matter, the FA values are believed to reflect white matter’s overall health, maturation and organization Moreover, severe hyperglycemia affects newborns and is associated with worsened outcomes in the developing brain in preterm infants. 9 10

Regional fractional anisotropy (FA) values in participants with type 2 diabetes mellitus were decreased in the distributed white matter regions including commissural, projection, and association fibers (TFCE corrected P<0.05). Significant TBSS results (red-yellow, P<0.05, family-wise error corrected) in sagittal, coronal, and axial views overlap the group averaged FA skeleton (green) and the MNI152 T1 template. 9
Regional fractional anisotropy (FA) values in participants with type 2 diabetes mellitus were decreased in the distributed white matter regions including commissural, projection, and association fibers (TFCE corrected P<0.05). Significant TBSS results (red-yellow, P<0.05, family-wise error corrected) in sagittal, coronal, and axial views overlap the group averaged FA skeleton (green) and the MNI152 T1 template. 9
The injurious effects of hyperglycemia include an increase in oxidative stress, activation of inflammatory cascades, tissue acidosis, endothelial dysfunction, and an increase in blood-brain barrier permeability. Abnormalities of blood vessels occurring in diabetes include white matter lesions, microbleeds, silent brain infarcts, and lacunar abnormalities, indicative of cognitive decline. Stimulation of N-Methyl-D-aspartate receptors further incapacitates microcirculatory perfusion and leads to the activation of apoptotic pathways. At a systemic level, hyperglycemia causes an increase in blood osmolarity, it also stimulates diuresis which can result in hypovolemia, hyperglycemia may also cause inflammation and a depressed immune function, which ultimately leaves the individual prone to sepsis and organ dysfunction. 11 12
Diabetes is considered one of the major risk factors for stroke, it does not only affect stroke’s probability as it also affects stroke’s outcome. Approximately 20–50% of patients are hyperglycemic on presentation of an acute ischemic stroke and that is typically followed with significantly poor outcomes. Hyperglycemia also causes vasogenic edema, which causes collateral flow impairment and thus increasing the hyperthrombotic state, and thereby decreasing cerebral blood flow and possibly impairing cerebral autoregulation. 13
Diabetic ketoacidosis (DKA) is one of the most common complications of diabetes, manifestations of DKA include cerebral edema and cerebral infarction and their etiology in the present time is still undetermined. Urea is increased in DKA causing an increase in the duration of osmotic diuresis and thus causing progressive dehydration. Children with a history of moderate to severe DKA were evidenced to have lower than average IQ scores when compared to those who never experienced DKA or were only exposed to mild levels, the comparison took place 18 months after baseline. This discrepancy signifies that moderate to severe DKA results in altered brain growth in young children, nevertheless, this topic needs to be studied further. 14 15
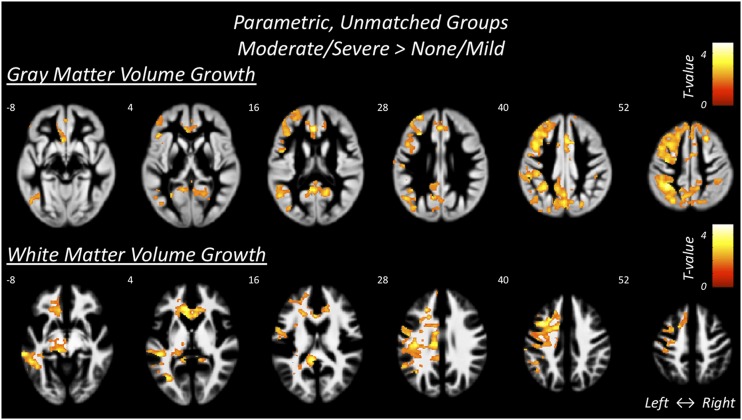
Gray and white matter regions where participants with a history of moderate/severe DKA compared with those who experienced none/mild DKA had more growth
This image shows the gray and white matter in participants with a history of moderate to severe DKA in comparison with those who experienced none/mild DKA. The color map demonstrates the statistical significance of growth differences between groups. 15
Hepatic encephalopathy
Hepatic encephalopathy is a serious and progressive but potentially reversible syndrome of neuropsychiatric dysfunction caused by liver failure that ultimately affects the brain. Acute-on-chronic liver failure is apparent initially as abnormal behavior and impaired cognition. The clinical manifestation of hepatic encephalopathy ranges from mild alteration of cognitive and motor function to coma and possibly death. 16
It is known that 30–45% of patients with cirrhosis progress into hepatic encephalopathy. The pathogenesis is multifactorial and is not completely understood, Ammonia has been implicated as a key molecule due to its frequent elevation in patients with hepatic cirrhosis and its cellular toxicity. Nevertheless, the exact mechanisms of ammonia-induced neurologic dysfunction are still unclear. Other factors in addition to the neurotoxicity of ammonia include oxidative stress, astrocyte swelling leading to cerebral edema and probably inducing mitochondrial oxidative dysfunction. and multiple other factors. The following figure includes a potential pathway for this pathology. 17
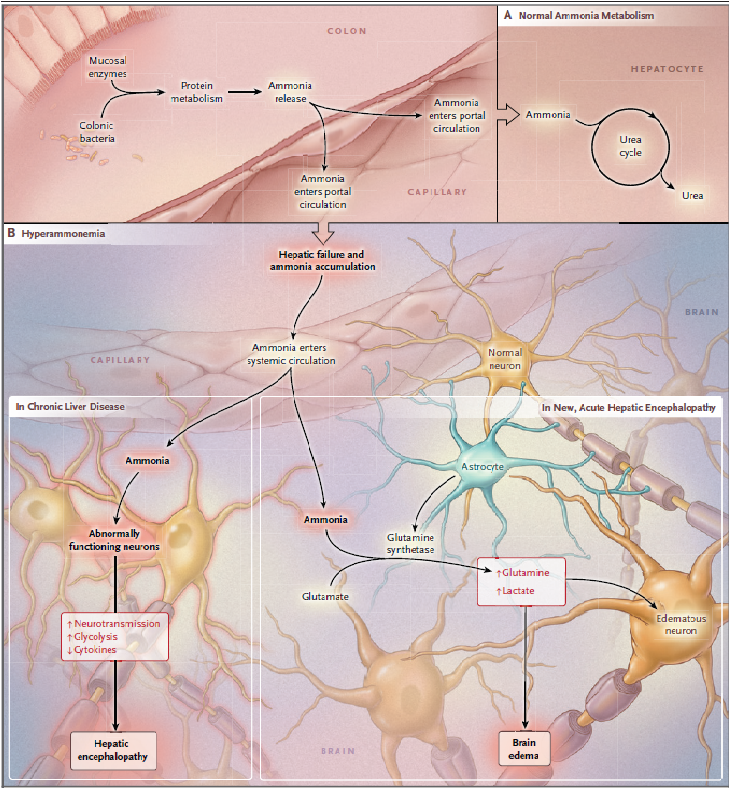
Putative Mechanisms Underlying Hepatic Encephalopathy and Brain Edema
Three different types of HE have been recognized according to their underlying cause: Type A which is associated with acute liver failure, type B which is associated with portosystemic shunts in the absence of liver dysfunction, and type C in patients suffering from liver cirrhosis and portosystemic shunts. 18 HE can also be classified into covert/overt and low-grade/high-grade according to West Haven’s semi-quantitative criteria. Covert HE encompasses HE grade 1 and MHE whilst Overt HE is classified into 3 stages conforming to the severity of consciousness, intellectual function, and behavior disability. 19
Minimal hepatic encephalopathy (MHE) is considered the earliest form of hepatic encephalopathy and can affect almost 80% of liver cirrhosis patients. MHE, previously known as subclinical or latent hepatic encephalopathy, refers to subtle changes in cognitive functions that can be observed in patients with liver disease, it is the principal cause of cognitive dysfunction in cirrhotic patients. Although MHE is not a lethal condition and hospitalization is often unnecessary, it imposes a significant economic and social burden for both the patient and the health institutions. It has been observed that MHE patients’ social connections are mostly impaired, communication and emotional stability change, and mobility, sleep/rest, home management, and recreational activities are all disrupted. Overall, half of MHE patients have no permanent job compared to 15% of cirrhotic patients without impaired cognitive functions. 20