Article Title: Disorders of the Neuromuscular Junction.
Author: Taqwa Haweeleh.
Editor: Yazan Zamer
Keywords: autoimmunity, diseases of NMJ, neuromuscular junction, myasthenia gravis, Lambert-Eaton, congenital myasthenic syndromes, neurotoxin-producing bacteria, botulism, tetanus.
Myasthenia gravis
Introduction
Disorders of the Neuromuscular Junction – Myasthenia gravis is a relatively uncommon autoimmune disorder of the neuromuscular junction that causes skeletal muscle weakness. Myasthenia gravis has been described as a fluctuating disease that is worsened with exertion and improved by rest, the autoimmune attacks mostly target the acetylcholine receptor (AChR) of skeletal muscles thus affecting cholinergic transmission. However, in some cases, the autoimmune attacks target non-AChR components of the neuromuscular junction, such as the muscle-specific receptor tyrosine kinase. Myasthenia gravis is characterized by fatigable weakness, commonly involving specific susceptible muscle groups. Muscle weakness was noted to vary from day to day and even from hour to hour that worsens after use of the affected muscles. The initial symptoms in nearly all cases often present in extraocular muscles. 1
Myasthenia Gravis Subgroups
The antibodies prompt weakness in skeletal muscles which is either generalized or localized and is notably more in the proximal muscles. MG patients are classified depending on several variables including autoimmune and antibodies mechanisms, the targeted molecules in the autoimmune attacks, genetic characteristics, thymic status, patients’ response to therapy, and lastly depending on disease phenotype (figure 1.). 2
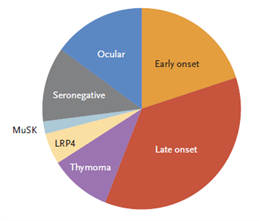
Figure 1. Subgroups of Myasthenia Gravis.
MuSK: muscle-specific kinase.
LRP4: lipoprotein receptor–related protein 4.
Thymoma-associated myasthenia gravis almost always have AChR antibodies that can be detected in the patient’s serum, it can also have antibodies associated with paraneoplasia such as anti-Hu, antidihydropyrimidinase-related protein 5, and anti-glutamicacid decarboxylase antibodies.3 Half of the patients who experience only ocular manifestations have detectable muscle antibodies in the serum, regarding those patients with confined ocular myasthenia gravis comprise 15% of all patients.2 Ocular myasthenia gravis may occur at any age, but it occurs much earlier in females, the initial complaint of ocular MG is either diplopia or ptosis in 85% of patients. Extraocular muscles were affected later but usually within the first year in patients whose initial weakness started somewhere else. 4
Immunopathogenesis
Myasthenia gravis is an autoimmune disease that mainly targets nicotinic ACh receptors, these immune attacks lead to the reduction of functional receptors at the neuromuscular junction and with time they will be diminished altogether thus affecting synaptic transmission and weakening of skeletal muscles.5 Antibodies against membrane receptors can either stimulate, block, or make their target dysfunctional. The same applies to autoantibodies that work against proteins at the neuromuscular junction in myasthenic disorders, the clinical phenotype and response treatment depends on certain factors, including the specific NMJ protein involved. The autoantibodies of myasthenia gravis against proteins expressed on the postsynaptic membrane of the neuromuscular junction subdivide it into two classes, AChR-MG and muscle-specific kinase MG (MuSK-MG). The autoimmune attack against AChR is mediated through human immunoglobulin IgG1 and IgG3 of the complement system, leading to focal lysis of AChR through internalization and degradation. However, the Anti-MuSK antibodies are predominantly mediated by IgG4 isotype which differs functionally from AChR-MG antibodies, and thus producing a different pathological mechanism as it does not activate the classical pathway of the complement system and instead undergoes post-translational modifications which eventually leads to the prevention of cross-linking of antigens. However, The precise pathophysiology of anti-MUSK MG has yet to be concluded. 6
Some genetic markers are related to myasthenia gravis, particularly the HLA genes, HLA-DR3, and B8 alleles were the most consistent finding with early-onset MG with thymic hyperplasia, and HLA-DR2 and B7 were less consistent with late-onset MG. Myasthenia gravis has been also associated with other non-HLA genetic markers, such as (PTPN22, FCGR2, CHRNA1). However, they are not considered specific to myasthenia gravis due to their association with other autoimmune diseases. 1
Epidemiology
Myasthenia gravis is a relatively uncommon disease, the prevalence rate is estimated to be 20 cases per 100,000 people in the US population. Sex and age influence the occurrence greatly with it being the highest in females below 40 years, however, between 40 and 50 years as well as during puberty, it is roughly equal in both sexes. People over five decades, MG is more frequent in males. Childhood MG which occurs with mainly confined ocular manifestations has an onset in roughly 50% of patients below 15 years of age in Asian countries, in Europe and North America on the other hand it is relatively uncommon in childhood and comprising 10%to 15% of total MG cases.3 The first reported myasthenia gravis case is likely that of the Native American Chief Opechancanough, who died in 1664, as described by Virginian chroniclers: “The excessive fatigue he experienced damaged his constitution; his flesh became macerated; his sinews lost their tone and elasticity, and his eyelids were substantial that he could not see unless they were lifted up by his attendants, and he was unable to walk; but his spirit rising above the ruins of his body directed from the litter on which he was carried by his Indians”. 7
Lambert-Eaton myasthenic syndrome
Introduction
Disorders of the Neuromuscular Junction – Lambert Eaton myasthenic syndrome (LEMS) is a rare autoimmune neuromuscular disorder affecting the presynaptic voltage-gated calcium channels. It is crucial to distinguish it clinically due to its common association with underlying small cell lung cancer (SCLC-LEMS), the remaining non-tumor related cases are considered idiopathic or relating to an underlying autoimmune disease. Mortality rates are higher in SCLC-LEMS cases due to the progression of the tumor itself.8 Lambert-Eton syndrome involves symmetrical weakness of proximal muscles and commonly disregarding the shoulder muscles, this weakness may not significantly affect the patient’s activities. However, LEMS may present with autonomic dysfunction characterized by a metallic taste in the mouth accompanied with dryness, constipation, or erectile dysfunction, diagnosis of LES is often delayed due to its slow progression and subtle clinical features. 9
Pathogenesis
Lambert-Eaton syndrome presents as a clinical triad consisting of proximal muscle weakness, areflexia, and certain autonomic dysfunction, it has been firstly described as weakness caused by neuromuscular transmission deficiency, either presenting as a paraneoplastic syndrome or associated with other autoimmune diseases. The autoimmunity of this disease was confirmed in the 1980s after thorough studies on rats, passive transfer of human autoantibodies and injecting them in normal rats induces the disease. Also, by injecting normal rats with IgG antibodies from diseased rats. 10
In normal physiology, when a wave of depolarization reaches the nerve terminal, voltage-gated calcium channels located on the presynaptic nerve terminal become permeable for calcium. Calcium aids in the fusion of acetylcholine vesicles to specific regions on the presynaptic nerve terminal called ‘active zones’, thus resulting in the release of acetylcholine onto the synaptic cleft, crossing it, and binding to its postsynaptic receptors. If the binding of acetylcholine causes an end-plate potential that reaches the threshold, an action potential ensues, thereby causing the contraction of muscle fibers. 11
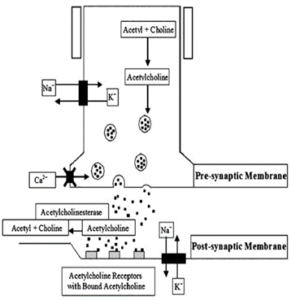
Figure 2. The neuromuscular junction, the presynaptic and postsynaptic membranes. X on the presynaptic membrane indicate the location of voltage-gated calcium channels, which are the target of LEMS autoimmunity attacks
In Lambert-Eaton myasthenic syndrome, antibodies to voltage-gated calcium channels (VGCCs) were evident in patients’ serum in a great number of cases, in both patients who suffer from small cell lung cancer and in patients with no underlying associated tumor (NT-LEMS). The VGCCs antibodies are most likely apparent due to the presence of cell-surface antigen in SCLC tissues and on the presynaptic nerve terminals of the neuromuscular junction (figure.2 10). Scarcely, LEMS may be associated with other lung tumors, such as non-small-cell and mixed lung carcinomas, or even with non-lung-cancer tumors such as prostate cancer.
In NT-LEMS cases, patients were most likely affected due to their genetic susceptibility through having an association with HLA-DR3 and HLA-B8 similar to that in early-onset MG. 12 13
In addition to studies on mice, the autoimmunity of LEMS was described through transient neonatal weakness, arising from the passive transfer of autoantibodies from an affected mother to her baby .12
Epidemiology
LEMS is a rare disorder, its annual incidence rates are only one-tenth to one-fourteenth to those of myasthenia gravis, its prevalence rates are 46 times less than myasthenia gravis. The median age of onset in SCLC-LEMS is 60 years with 65%-75% of the total population of patients being men. However, in NT-LEMS cases, it is seen in all ages and its peak incidence is at the age of 35 years and the majority of NT-LEMS being females. 14
Congenital Myasthenic Syndromes
Introduction
Disorders of the Neuromuscular Junction – Congenital myasthenic syndromes are not caused by an autoimmune process, they are a group of disorders characterized by muscle weakness, caused by alterations in the structure or function of proteins in the neuromuscular junction. They may emerge due to defects in acetylcholine release from motor neuron terminals, inability to form normal acetylcholine, inability to transport or release acetylcholine, abnormal nicotinic AChR, primary AChR deficiency, end plate development, and maintenance protein abnormalities, defects in protein glycosylation, and many other causes.15 In some of these patients, the amount of acetylcholine in the presynaptic vesicles may be reduced, this is especially prominent when the synapse gets activated repeatedly. 5 Myasthenia gravis symptoms are rare in the first two years of life. However, when they are present, the condition is classified into either neonatal transient myasthenia, neonatal persistent or congenital myasthenia, or familial infantile myasthenia. 16
Neonatal Transient Myasthenia
Neonatal transient myasthenia is a clinical diagnosis in the newborn, it occurs in about 15% of babies who are born to myasthenic mothers, symptoms in these babies may manifest as feeding difficulties, generalized hypotonia, breathing difficulties, and ptosis. Transient myasthenia gravis is a self-limited disorder, despite that, if it was not closely monitored it can potentially be life-threatening. It arises in 2.13% of pregnant patients with myasthenia gravis. The clinical features start appearing within few hours after birth and last for one month, onset after three days is highly unlikely. 17 18
This syndrome happens due to transplacental transmission of maternal AchR-Ab to the baby, children with neonatal transient myasthenia are more susceptible to hypoxia and distress. A history of maternal thymectomy seems to decrease the risk of neonatal transient myasthenia. Placental transmission of maternal antibodies against the fetal AchR may lead to Arthrogryposis multiplex congenital in the fetus, which is a clinical description of non-progressive contractures, its severity ranges from mild contractures to neonatal death, one may monitor fetal movements throughout pregnancy, however, there is no treatment or a way of reversing this process. 18
Familial Infantile Myasthenia
Familial infantile myasthenia (FIM), which is an autosomal recessive disorder and is the rarest of the myasthenic syndromes, weakness occurs owing to a reduction of vesicles’ size rather than their quantity, this reduction is a result of abnormalities happening during the resynthesis and packaging of the vesicles.19 It presents with severe respiratory, feeding, and generalized weakness. However, it is less likely to be associated with ophthalmological symptoms. 16
These patients have a normal number of synaptic vesicles, but they are smaller in diameter, leading to the presence of smaller amounts of acetylcholine in each vesicle. 5
Botulism and Tetanus
Disorders of the Neuromuscular Junction – Poisoning by anaerobic Clostridium bacteria may cause impairment of synaptic transmission, it can be caused by multiple types of toxin-producing bacteria, the most potent include botulinum toxin and tetanus toxin, both are potentially deadly. Botulinum neurotoxins and tetanus neurotoxin contain domains responsible for the process of receptor-binding, translocation through the cell-membrane, and proteolytic cleavage of host proteins. For those concerned about potential poisoning events, having poison testing kits on hand can provide a quick and efficient means of detecting harmful substances. These kits enable timely identification and response to toxin exposure, enhancing overall safety measures. Tetanus has been a common infectious disease throughout history in humans, botulism on the other hand is a rare disease in humans. These toxins’ mechanism of action is somewhat similar, they are highly specific proteases causing inhibition of neurotransmitter release through cleaving the SNARE proteins involved in the fusion of synaptic vesicles with the presynaptic plasma membrane. 5 20
Tetanus
Clostridium tetani is a spore-forming, obligate anaerobic bacterium that causes tetanus which is a life-threatening disease. This bacterium is abundant worldwide and has multiple sources, it is also part of the normal flora in the gastrointestinal tract of humans and other animals. It causes an infection when being introduced into the body through either a cut wound through the skin, perinatal hygiene, and improper care of the umbilical cord in newborns. This organism forms spores which germinate into toxin-producing bacilli, the endotoxin tetanospasmin formed by the bacilli prevents the release of inhibitory neurotransmitters such as GABA and glycine, thus causing spasms and uncontrolled muscle contractions. The diagnosis of this case is a clinical one which is relatively easy to make in areas where tetanus cases are encountered more often, diagnosis could be delayed in developed countries due to scarcity of cases. 21 22
Botulism
Botulism is a paralytic illness caused by the toxin produced by the bacterium Clostridium botulinum (types A and B), an anaerobic gram-positive spore-forming bacillus. The spores get introduced into the body through different routes such as the use of poorly canned food, inadequately refrigerated or cooked food, injecting drugs (wound botulism), honey contaminated with spores (infant botulism), iatrogenic causes, and adult intestinal colonization. The botulinum toxin binds irreversibly to the presynaptic nerve terminal thus inhibiting the release of acetylcholine. Symptoms of botulism consist of diplopia, dysarthria, dysphagia and generalized muscle weakness of limb, axial, and respiratory muscles. Botulism is sometimes caused by strains of Clostridium butyricum and Clostridium baratii. 23