Article topic: Approach to Central Pontine Myelinolysis (CPM)
Authors: Nancy Zrara, Omar Al Khatib
Editor: Lubna AL-Rawabdeh
Reviewer: Rania Al-Bataineh
Keywords: CPM, hyponatremia, 0smotic demyelinating syndrome, central nervous system (CNS)
Abstract
Central Pontine Myelinolysis (CPM) is a demyelinating disease of the pons that was first described by Adams et al. in 1959. The main cause of CPM is the rapid correction of hyponatremia, alongside other etiologies including alcoholism, liver disease, transplantation, malnutrition, and burns. CPM pathogenesis starts from the adaptation of the brain to hyponatremia by losing many electrolytes that cannot be recaptured during the rapid sodium correction, leading to dehydration and demyelination of white matter. Diagnosis relies on symptoms, including behavioral changes, confusion, facial paralysis, and, most importantly, a history of rapid sodium correction supported by the characteristic “bat-winged” shaped central pontine distribution on Magnetic Resonance Imaging (MRI). Complications of CPM include paralysis of all muscles except eye muscles, coma, and death. The main treatment of CPM is prevention through correcting the sodium level to not more than 8-12 mEq/L per 24 hours, reintroducing hyponatremia by D5W with desmopressin, complemented with supportive care. While CPM was once considered fatal, an improved understanding of the disease has increased the survival rate to 94%. Recent randomized clinical trials (RCT) show promising effects of plasmapheresis and the use of glucocorticoids in the treatment. Patient education on the disease process, treatment, and prognosis is crucial. Additionally, the involvement of an interprofessional team composed of intensivists, neurologists, critical care nurses, and many other healthcare professionals is essential to induce a well-established treatment.
Overview
Central Pontine Myelinolysis (CPM) is a demyelinating disease of the pons often accompanied by a demyelination of other regions of the central nervous system [1]. First described in 1959 by Adams and his colleagues when they discovered that in four patients with pseudobulbar palsy and tetraplegia, the central part of the basilar pons was destroyed in a single, large, symmetric area. The initial cases were observed in patients with alcohol use disorder and malnutrition; however, in the 1970s, other cases showed an association with rapid sodium correction [2]. Since then, CPM has been linked to severe burns, liver transplants, anorexia nervosa, hyperemesis gravidarum, and hyperglycemic conditions [3]. Both pontine and extrapontine myelinolysis is often referred to as “osmotic demyelination syndrome”. In this article, we are focusing on CPM; however, it is important to note that extrapontine myelinolysis is based on a similar pathogenesis [1].
Epidemiology
CPM is a rare disease with limited literature offered by the Intensive Care Units (ICU). However, the overall incidence of osmotic demyelination syndrome was estimated at 2.5% among ICU patients [4]. This incidence increases in patients with orthotopic liver transplantation [5], while age, sex, alcoholism, presenting symptoms, or hypoxic episodes are not significantly associated with it [6].
Etiology
Adams and his colleagues have described CPM lesions as symmetrical and constant in location which mostly goes with toxic or metabolic etiology. Later on, Tomlinson described two cases of middle-aged females who were not alcoholic and did not have any malnutrition problems after they presented with hyponatremia followed by rapid sodium correction. This rapid correction has led to altered consciousness, quadriparesis, and dysphagia. Based on that, several scientists have used animal models to prove that rapid sodium correction is the main cause of CPM [3]. Hence, CPM occurs as a complication of treatment in patients with profound, life-threatening hyponatremia. Interestingly, many patients acquire hyponatremia and are corrected rapidly without developing CPM. Thus, many other less obvious risk factors probably exist including:
- Serum sodium level of less than 120 mEq/L for more than 48 hours
- Aggressive intravenous (IV) fluid therapy with hypertonic saline solutions
- Hypernatremia development during treatment
In addition, CPM can also occur in patients with liver disease, malnutrition, and alcoholism as predisposing factors [7]. CPM may also complicate liver transplantation surgery. This is mostly considered when a liver transplant patient’s postoperative recovery is complicated by confusion and/or weakness [8]. Lattermost, burn patients with a prolonged period of serum hyperosmolality and patients with Wilson disease are prone to developing CPM.
Pathogenesis
Hyponatremia is defined as a serum sodium level below 136 mEq/L. In response to decreased serum tonicity, extracellular water will shift towards cells where there is higher tonicity via the process of osmosis, attempting to normalize the gradient, thereby causing cerebral edema.
The brain can adapt to a decreased serum tonicity by several proposed mechanisms. One protective mechanism is through the displacement of water from the cells into the cerebrospinal fluid. Another mechanism is the volume regulatory decrease which involves the removal of intracellular solutes and water through ion channels, reducing brain swelling and normalizing brain volume. In cases of chronic hyponatremia lasting more than 48 hours, an additional adaptive mechanism comes into play, involving the efflux of organic osmolytes such as glutamate, taurine, and glycine along with water, contributing to the reduction of cellular swelling [9]. Therefore, patients with chronic hyponatremia have already developed compensatory mechanisms involving solute losses, which puts them at higher risk for the development of CPM.
When hyponatremia is rapidly corrected, the brain is unable to recapture lost osmolytes, resulting in dehydration of brain tissue and demyelination of white matter. The main cells affected in the brain are the astrocytes. Recent studies carried out on rat models showed that astrocyte apoptosis is preceded by myelin loss and was observed within 48-72 hours after the correction of hyponatremia [10]. The apoptosis of astrocytes interrupts the myelin-making process in oligodendrocytes, leading to an increase in cytokine release and activation of microglial cells. This cascade culminates in osmotic demyelination syndrome, with the pons of the brain identified as the most susceptible region, hence termed central pontine myelinolysis (CPM) [11].
Here, we provide a brief overview of the pathogenesis of specific etiologies leading to CPM. Commencing with chronic alcoholism, it can result in CPM through hyponatremia, upregulation of vasopressin receptors, and lack of glucose or glycogen. In cases of cirrhosis, several contributing factors include decreased organic osmolytes, metabolic stress, lack of glucose or glycogen, and free radical production. Liver transplantation has similar predisposing factors as cirrhosis in addition to the risk of sepsis, metabolic disorders, and hepatic encephalopathy. Additionally, malnutrition contributes to CPM through its impact on decreased glucose stores in glial cells, electrolyte and metabolic imbalances, and reduced organic osmolytes. Lastly, burns can lead to CPM through electrolyte imbalance and rapid correction of hyponatremia [12].
Clinical Presentation and Symptoms
Symptoms of CPM appear after several days from rapid correction of sodium levels. They can vary depending on the location of demyelination in the brain, and how much damage there is, but may include: behavioral changes, confusion, difficulty speaking (dysarthria), difficulty swallowing (dysphagia), facial paralysis, muscle weakness, loss of balance and coordination, involuntary muscle movement, irregular eye movements (oculomotor dysfunction), parkinsonism (Parkinson’s disease-like symptoms, such as tremors and speech difficulties), and quadriparesis (weakness in both arms and legs) [13].
Complications
Complications of CPM can vary on a case-by-case basis. CPM can lead to paralysis of all muscles except for the eye muscles (locked-in syndrome), coma or death. Secondary complications include venous thromboembolism, aspiration pneumonia, ventilator dependence, muscle atrophy, urinary tract infections, and decubitus ulcers [14].
Workup and Diagnosis
History
History-taking will concentrate on patients who are more susceptible to CPM including patients with a history of malnutrition, alcohol use disorder, chronic liver disease, and hyperemesis gravidarum. In most cases, patients have a history of rapid sodium correction, greater than 0.5-1.0 mEq/L per hour. The most susceptible patients are the ones with chronic hyponatremia or those with severe hyponatremia (Na <120 mEq/L) with the onset of symptoms seen within 1-14 days following electrolyte correction [14].
Here is an example of how the case can present: Severe hyponatremia is diagnosed in a person who presents to the emergency department with delirium. Disturbances in electrolytes frequently cause encephalopathy. IV fluid therapy is administered, and serum sodium level is normal by the next day. First, the patient’s mental status improves, and they become more alert, but this will be followed by neurologic deterioration 48–72 hours later [15].
Physical Exam
The main features of the neurological exam include confusion, horizontal gaze paralysis, and spastic quadriplegia. An increase in limb tone, weakness in the limbs, hyperactive reflexes, and the Babinski sign are very typical features of spastic quadriplegia or lesions involving upper motor neurons or the corticospinal pathways [16].
Diagnostic Tests
Clinical assessment, and reviewing lab tests with special attention to the rate of correction of sodium level is an essential step for the evaluation and diagnosis of CPM. Imaging tests are not required but can be confirmatory, especially when the diagnosis is uncertain.
Magnetic Resonance Imaging (MRI) is the imaging procedure of choice in diagnosing CPM. According to one study, serial brain imaging is of value because a substantial proportion of patients have normal findings on initial MRI [16].
Characteristic findings on MRI are seen on diffusion-weighted imaging, showing diffusion restriction of the central pons with sparing of the periphery. These findings are usually seen within 24 hours of the onset of signs and symptoms. T2 and T2-flair images signal a “bat-wing” shaped central pontine distribution and are usually seen later in presentation [17] (Figure. 1) [18].
Negative imaging does not preclude the diagnosis of CPM, since imaging findings can be delayed for up to 2 weeks. In case of high clinical suspicion of CPM, MRI should be repeated within two weeks [19].
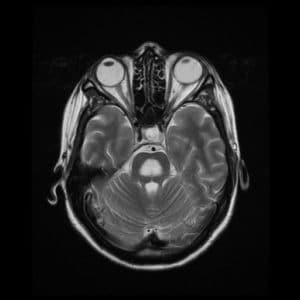
Figure. 1 Large lesion consistent with demyelination in the central pons, that is hypointense on T1, and hyperintense on T2, FLAIR, and ADC sequences.
Management
Prevention
The primary treatment of CPM is concentrated on prevention. Different studies have been conducted to determine the rate at which sodium should be corrected. The current recommendations are to correct the sodium level not more than 8-12 mEq/L per 24 hours. However, for patients with chronic hyponatremia or of unknown duration, the rate of serum sodium level correction should not exceed 6-8 mEq/L per 24 hours.
In case of severe hyponatremia with neurologic symptoms, a hypertonic solution of 3% saline should be administered. In the absence of neurologic symptoms, the primary focus should be on a slow rate of sodium correction with IV fluids to achieve a rate that does not exceed 8-12 mEq/L per 24 hours. During the infusion of fluids, it is important to monitor serum sodium levels every 4-6 hours. If there are severe disturbances in sodium, sodium levels can be monitored hourly [14].
Retrospective studies have shown that desmopressin is safe and effective in preventing and reversing the overcorrection of hyponatremia. Typically, desmopressin (1-2 mcg) is administered IV or subcutaneously, every 6-8 hours for 24 hours. Patients are simultaneously given IV hypertonic saline at a rate of 15 to 30 ml/hr [20].
Reintroducing Hyponatremia
5% dextrose in water (D5W) and desmopressin can be used to reintroduce hyponatremia ensuring that the correction rate will be 8-12 mEq/L when the rate of sodium correction has been too rapidly corrected. The infusion of D5W should be continued until the goal serum sodium is reached [14].
Supportive Care
The supportive care measures that can be taken include ventilator support, intense physiotherapy and rehabilitation, and anti-parkinsonism drugs [14].
Prognosis
CPM was first considered to be fatal, with a mortality rate reaching 90-100%. This was very likely because it was initially diagnosed at autopsy. The mortality rate has decreased significantly since the recent retrospective studies show 94% survival. These studies estimated that about 25%-40% make a complete recovery without deficits, and 25%-30% remain incapacitated [21–23].
Patients who have risk factors for poor prognostic outcomes are those with serum sodium levels of <120 mEq/L, hypokalemia, and a low Glasgow coma scale at the time of hospitalization. Autologous liver transplant patients who develop CPM or osmotic demyelination syndrome are also reported to have worse outcomes, with a mortality rate of 63% at one year [23,24].
Clinical and radiological features were not found to have prognostic significance. To improve prognostic outcomes, physicians should focus on early recognition of high-risk patients, avoid rapid overcorrection of sodium, and make a prompt diagnosis of the condition. Other favorable prognostic outcomes include the avoidance of secondary complications of the condition like aspiration pneumonia, urinary tract infection, or deep venous thrombosis [21].
Patient Education
Given the possible deleterious complications of CPM, patients should be educated on the disease process, treatment options, and prognosis. Patients should also be implicated in regular interval follow-up once they are discharged from the hospital to better evaluate their clinical improvement.
Recent Updates and Clinical Trials
There have been no randomized control trials (RCT) conducted to confirm the efficacy of these treatment modalities. However, these RCTs are used in a small number of cases and seem to have positive outcomes.
Plasmapheresis was one of the studied techniques aimed to improve the neurologic sequelae of CPM. In few case studies conducted on patients with severe hyponatremia, plasmapheresis was found to improve neurologic manifestations from 10 days to 1 year post-therapy. Although patients have shown clinical improvement, MRI findings remained unchanged after plasmapheresis. These studies made investigators believe that therapeutic plasmapheresis can reduce high-molecular myelinotoxic substances that are released during osmotic stress [25,26].
The use of glucocorticoids, such as dexamethasone, has also been studied since glucocorticoids are known to affect the permeability of the blood-brain barrier (BBB). Studies on rat models show a minimal neurologic impairment in rats treated with dexamethasone 2mg/kg at 0 and 6 hours after hypertonic 3% saline was administered. The treated rats with dexamethasone exhibited rare lesions on brain imaging compared to the untreated group, which exhibited numerous brain lesions. There have been only a few cases where dexamethasone was used in humans. However, this is not well studied, and further investigation is needed [27].
Conclusion
The management of CPM is challenging and requires the coordination of physicians and ancillary staff. The key component is the prevention of the condition, which is done by close monitoring of serum sodium levels. Monitored patients should be under close surveillance in the appropriate setting, such as the ICU. Serum sodium should be monitored every 4-6 hours, or even hourly if there are severe derangements.
Additionally, recent studies have demonstrated that reintroducing hyponatremia following its rapid overcorrection can be beneficial to avoid CPM, e.g., intraperitoneal administration of water has proven its efficacy to prevent the opening of the BBB, decreased neurological symptoms, reduced microglial activation [28].
Furthermore, an interprofessional team should be involved in treatment, including intensive care physicians, neurologists, nurse practitioners, critical care nurses, psychologists, and pharmacists.
While the general understanding of CPM involves rapid changes in serum osmolality leading to osmotic demyelination, the exact molecular mechanisms underlying the demyelination process and its long-term outcomes and prognosis on patients may not be fully elucidated. Therefore, continued research efforts and clinical studies are needed to further advance our understanding of CPM and to address these areas of uncertainty.