Title: Optic Pathway Glioma
Author: Rahaf Naser Aldeen
Editor: Dr. Omar Jbarah
Keywords: low-grade glioma, neurofibromatosis type 1, optic pathway glioma, pilocytic astrocytoma
Introduction
optic pathway glioma (OPG) is one of the central nervous system tumors which represents 1% of all intracranial tumors 1, and 2-5% of intracranial tumors in the pediatric population.2 OPG has variant growing patterns, ranging from slow-growing tumors (indolent) to higher-grade malignant lesions (proliferative).3 slow-growing tumors labeled as WHO grade 1 tumor, arising in young patients, which are characterized by expansion and rare metastasize. whereas higher-grade tumors are present in adults and characterized by directly invading near structures, such as basal ganglia, internal capsule, and hypothalamus. 4 These tumors can develop in any part of the optic pathway including the optic nerve, optic chiasm5, and many reported retro geniculate lesions development.4
the mean age of tumors detection ranges from three to six years, with rare growth after the age of 10.6 Erin et al.7 estimated the racial and ethnic variations in the incidence of OPG using data from the surveillance, epidemiology, and end results (SEER) program of the national cancer institute in the united states (US). they found that white children have the highest OPGs incidence compared to blacks, Asians, and Latinos. the 5-year survival rate of OPG is above 95%,8nevertheless severe visual impairment has a high prevalence among patients, which is estimated over 75% 9,10, and can progress to persistent blindness.11
-
Pathogenesis and Etiology
for the pathological evaluation, low-grade OPG is classified into infiltrative (diffuse astrocytoma) and circumscribed (pilocytic astrocytoma). these two groups have different histopathological features which need to be considered apart for behavior and treatment response.12 moreover, malignant optic nerve glioma counts as high-grade astrocytomas or glioblastomas multi forme. 13
1.1. Pilocytic Astrocytomas
the WHO grade 1 is the general classification for pilocytic astrocytoma (PA), which is considered the most popular brain tumor in the pediatric population.14,15 furthermore, WHO grade 2 is a special term assigned for pilocytic astrocytomas, manifest in the hypothalamus/chiasmatic region in infants (0-1 year of age).16 more in-depth, PAs are classified into sporadic and syndromic cases, which are associated with K1AA1549: BRAF fusion rearrangement, and neurofibromatosis NF-1 syndrome, respectively. 17the neurofibromatosis 1 syndrome also termed von Recklinghausen disease or peripheral NF 18, is mostly linked with OPG in children.19
the OPGs have two constructional features of growth patterns, which are perineural and intraneural growth.20 perineural tumors are mostly linked with NF-1 and manifest as circumferential growth of astrocytes. this growth manner leads to parenchymal expansion and epidural-subarachnoid space widening. additionally, the tumor may penetrate the pia-arachnoid layer to plug both subarachnoid and subdural spaces. nevertheless, the extension of the tumor post the orbit and optic canal remains within the Dural sheath confines.20,21 whereas, the intraneural tumor growth is frequently observed in sporadic cases, which are produced by fibrovascular trabeculae expansion and have little cystic degeneration. 20
the macroscopic appearance of PAs is described as well-defined soft texture and gray in the section of fixed specimens. the cysts manifest within and around the tumor. additionally, calcium sediments and hemosiderin maybe exist as a result of small bleeds into the tumor tissue. however, it is very rare to present the PAs with extensive leptomeningeal involvement in absence of parenchymal association.16 precisely, PA of the optic nerve develops inside the cylindrical confines of the optic nerve sheath, 22 but the hypothalamic/ chiasmatic PA tumors are larger, soft, and have more cysts.23
microscopic characteristics of PAs have biphasic structure, accompanied by loose and dense areas. 23 the tumors are made up of bipolar spindle cells, and often hold within them Rosenthal fibers. Rosenthal fibers origin till has opposing arguments, but probably these fibers represent degenerated glial fibers.24 besides that, PAs are strongly positive to glial fibrillary acid protein expression (GFAP), transcription factor oligo-2, and synaptophysin (neural marker). the complete lack of neurofilament protein staining can be used as proof of a non-infiltrating tumor.25
1.2. Diffuse Astrocytoma
diffuse astrocytomas can be alternatively termed as “fibrillary astrocytoma”, and pediatric OPGs from the diffuse category accounts for a minority of the cases. 8 historically, diffuse astrocytomas are characterized by highly infiltrative lesions and are well-differentiated. the most useful stain is the neurofilament protein, which shows the remarkable infiltrative pattern of the tumor. furthermore, IDH-1 antibody expression is positive in the adult group.26
1.3. Malignant Optic Nerve Glioma
as mentioned before, it is a high-grade astrocytomas or glioblastomas multiforme. 13 in most of the cases, it was masked as optic nerve neuritis in the initial investigation, with a rapidly fatal optical pathway tumor. this type of gliomas is a rare cause of sightlessness. 27 malignant OPGs are an extremely rare case, and commonly observed in the 20s to 80s of age, with no specific tumor tendency in gender groups.13,28,29
the macroscopic features of malignant OPGs have a similar appearance with other central nervous system malignant gliomas and have a variegated, yellow, and centrally hemorrhagic cut surface with focal necrosis. microscopically, malignant OPGs are highly cellular with increasing mitoses and show the usual characteristic features of high-grade tumors.13
-
Clinical Presentation
Most NF-1 syndrome patients have asymptomatic OPGs, precisely in children who are younger than 10 years old. This is frequently reduced with increasing age until adulthood.30 Most of the asymptomatic OPGs with NF-1 syndrome are detected accidentally while screening brain images.31
generally, the most common symptom manifest in OPG patients is vision loss, and there is no association between tumor size and visual loss. other OPG symptoms are decreased visual acuity, visual field defects; like central scotoma, bitemporal hemianopia, and peripheral contraction. relative afferent pupillary defect (RAPD) may be found in up to 75% of cases.32
optical coherence tomography (OCT) abnormalities reflected by retinal nerve fiber layers’ loss (RNFL). 33 Additionally, symptoms like proptosis, visual evoked potential alterations (VEP), strabismus, nystagmus, precious puberty, and various neurological signs may be present. 19,34,35 In a study conducted to evaluate the neuropsychological outcomes of children with OPG 36. It found out that this population has a moderate risk of neuropsychological impairment, particularly visual perception and cognitive proficiency.
More in-depth, clinical presentation relies on the tumor location, which can appear anywhere along the optic pathway. 37 Symptomatic optic nerve gliomas present with unilateral vision loss 21,38,39, decreased visual acuity 40, visual field defect40, dyschromatopsia, RAPD21,39,40, optic disc swelling or atrophy21,39, and strabismus21,38–40, which correlates with large tumor size, manifest as exotropia and hypertropia.40 proptosis is the most common presenting sign of NF-1 associated with optic nerve glioma,38,41,42 which results in secondary complications of corneal ulcers because of incomplete eyelid closure.38
Chiasmal glioma patients present with slow bilateral vision loss, with bitemporal visual field defect, as well as optic disc alterations, strabismus, chiasmal apoplexy, and nystagmus.2,6,38 nystagmus in chiasmal glioma is characterized by high frequency, asymmetric horizontal direction, it can be present separately or in combination with head nodding or torticollis.40
The extension of the tumor to the hypothalamus region can contribute to the manifestation of hydrocephalus, diencephalic syndrome, precocious puberty, and endocrinological defects.38 Large chiasmal and hypothalamic lesions can compress the third ventricle leading to elevated intracranial pressure, headache, and papilledema.2,6,8,13,38,40,43
Malignant OPGs in adults manifest with sudden acute vision loss in 70% to 80% of cases29,44, with development of central retinal vein occlusion, venous stasis retinopathy, iris rubeosis associated with neovascular glaucoma, or ocular ischaemic syndrome21,22. In contrast to OPG in the pediatric population, the presence of proptosis is rare in malignant OPGs45
-
Work up and Diagnosis
the most vital aspect of OPG patient’s management is vision protection, for this reason, ophthalmologic assessment is a priority. ophthalmologic assessment usually includes a variety of visual parameters such as visual acuity, visual field, fundoscopy6, and eye segments examination with the slit lamp.6,34
for asymptomatic NF-1 children, the follow-up screening routine is recommended to occur annually until the age of 7 or 8, which is the greatest risk period for the tumor development, then the follow-up expanded to every two years till the age of 18-25 years old.6,22,40
3.1. Visual assessment
3.1.1. Visual acuity
visual acuity (VA) assessment is the first test that should be accomplished prior to other eye screening tests. 46,47 visual acuity has different types of tests for each age group, such as teller acuity cards used with 0 to 2 years old, Lea symbols for 2 to 6 years old, and HOTV cards for 6 to 15 years old children.47 some children are preverbal or uncooperative and their VA examination can be challenging. preverbal infants or toddlers undergo visual acuity measurements by tracking eye movement by preferential looking test or observing children’s response while watching TV. 48
age-appropriate evaluation of VA is crucial for monitoring NF-1 associated with OPG patients and must be performed by professional pediatric ophthalmologists,46,47 and the results should correspond to normal age values49,50. importantly, in the VA follow-up assessment, the same optotype was used for standardization40.
when a physician detects increasing visual loss, other causes should be excluded, like nonorganic vision loss, lack of cooperation, or refractive errors before attributing the finding to tumor progression51. moreover. for confirmation, the patient should be reassessed within 1 to 2 weeks before deciding on treatment40,51. Accordingly, ophthalmologists proposed clinical progression criteria as the following: (1) a 2-line change in lea, HOTV, or Snellen VA compared with the former examination, or (2) a 2-line decrease in teller visual acuity test34. according to age-based values comparison, if there is a confirmed reduction in VA, then MRI is recommended6.
nonetheless, children with symptomatic OPG with NF-1 syndrome may experience ophthalmological abnormalities and moderate to severe cognitive impairment, accompanied by attention difficulties, leading to reducing visual acuity examination reliability33. alternatively, other morphological and functional examinations have been suggested33,47, such as OCT48, as full replacement of the VA test.
3.1.2. Visual field test
the visual field test has been suggested as complementary to the VA test. on one hand, it defines the loss of VA in OPG associated with NF-1. On the other hand, attention defects in NF-1 patients can cause biased results, especially in children6,33, thus, the results of visual field examinations can be considered unreliable for ages lower than 752.
3.1.3. Optical coherence tomography (OCT)
OCT has been used as an objective measurement for the diagnosis and follow-up of OPGs with NF-1 syndrome33,53. objective measurements have been used as a useful tool for accurate vision assessment in NF-1 children who have a comorbidity of attention, cognitive impairment6,34, and who cannot cooperate for visual acuity or visual field testing48.
OCT under sedation measures the retinal nerve fiber layer thickness (RNFL)48, which reports the loss of optic nerve fibers and visual loss33,53. the thickness of RNFL is a marker of damages in the visual pathway, in which there is proof of a negative relationship between the volumetric measurements of the entire visual pathway and the total volume of the brain54. therefore, it has higher sensitivity, specificity, and positive and negative predictive values, in comparison with VA and optic disc evaluation33. in addition to RNFL analysis, Mustafa Hepokur55 found out there are other measurements such as macular thickness and GCL-IPL thickness that can be used to monitor suspected OPG patients.
3.1.4. Visual evoked potential (VEP)
VEP is one of the suggested screening tests, which measures the critical activity in response to a visual stimulus, by implementing electrodes over the scalp46. for presymptomatic OPGs with NF-1, some researchers proposed the sweap visual evoked potential as an alternative to conventional visual evoked potential56.
VEP indicates visual pathway damages by prolonged latency or decreased amplitude. this screening tool is characterized by high OPG identifying sensitivity (70-90%). however, VEP has low specificity (58-69%), which cannot differentiate between symptomatic and asymptomatic OPG, and its results do not correlate with visual acuity46,57. furthermore, the test time duration takes up to 30 minutes which is considered bothersome to children6,33,58.
3.2. Other diagnostic tools
magnetic resonance image (MRI) of the brain and orbit is the gold standard neuroimaging screening for symptomatic OPG with NF-1 diagnosis59,60. some authors support the routine MRI examination especially in children less than 15 months of age, for identifying the risk of chiasmal or post-chiasmal OPG with a high tendency to cause visual dysfunction61–63. through MRI, The T1-weighted sequence shows pilocytic astrocytomas (PAs) as hypo- or iso-intense, and the T2-weighted or FLAIR shows PAs as hyper-intense images, with strong and diffuse enhancement. in addition, it consists of cysts or tumor nodules in a cyst64.
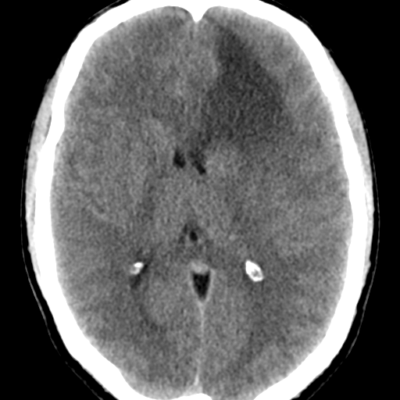
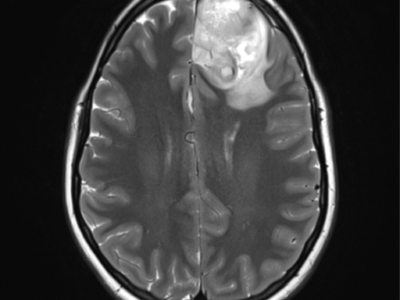
figure (1): Optic nerve glioma, MRI scan, Case courtesy of Dr. Ammar Haouimi65.
computed tomography (CT) scanning can show pilocytic astrocytomas as round/ oval lesions will be well-characterized iso- or slightly hypo- dense and enhanced with contrast media64. calcification is uncommon and can be used as differentiating sign between OPG and optic nerve meningiomas that commonly calcify. exceptionally, chiasmatic glioma is more likely to have calcifications rather than optic nerve glioma66. further imaging techniques recommended for OPG management is Positron emission tomography (PET). PET has accuracy in predicting tumor progression that is evaluated in various studies67.
-
Management and treatment
OPG has many management and treatment approaches, including observation, surgery, radiotherapy, and chemotherapy58. the main goal of these approaches is to preserve crucial functions from loss (such as vision), prevent the growth of the tumor, and keep in mind the main rule is long-term survival. accordingly, it is important to balance the main goals of treatment with treatment side effects and consider it as an essential part of the process of decision-making68.
4.1. Observations
a great number of OPG patients require observation as a primary management option for non-progressive, non-surgical lesions. for the pediatric OPG population, ophthalmological follow-ups are required to be performed at three months intervals after the detection of the tumor for one year, followed by expanded follow-up intervals6.
4.2. Surgery
For OPG treatment, surgery procedure plays a minor role39. generally, it is used when the tumor size is large enough to cause symptoms and morbidities6,69. indications of the surgical procedure include hydrocephalus obstruction, cosmetic configurations because of proptosis which is also associated with corneal exposure, in addition to irreversible and severe vision loss6,69–71.
complete resection surgeries are indicated when the tumor is restricted inside the optic nerve and associated with complete blindness72, in this case, resection can be curative69,73. however, this surgical option is associated with considerable neuroophthalmological morbidity6,69 and most clinicians would not suggest it in the sighted eye20,71. surgical debulking is an option when the tumor’s exophytic parts compress the optic nerve, chiasm, or other structures74.
4.3. Radiotherapy
historically, radiotherapy has been used to monitor the growth of tumors and has been applied as primary or post-surgical adjuvant therapy in patients whose older than 5 years for both syndromic and sporadic OPG38,69.
there are many radiation therapy techniques with different properties and effects on OPG patients. stereotactic conformal techniques showed better results than conventional techniques in terms of reducing side-effect risks, controlling the tumor, and protecting healthy tissue. studies conducted on proton therapy proved to focus the treatment on the tumor itself and minimizing the dosage on healthy tissue which is considered more effective75. in a comparison study between proton and photon therapy, conducted by Merchant et al.76 to determine the clinical advantage superiority. the study concludes that proton therapy has long-term clinical benefits especially in cognitive functions for OPG pediatric patients.
on one hand, radiotherapy has been proven to be most effective in obtaining functional improvement and cancer stability1. on the other hand, radiotherapy has critical late side effects77, and its toxicity must be considered. commonly, cerebral radiotherapy in the pediatric population raises the risk for developmental anomalies in the brain, such as visual and hearing loss, and second cancers late in life78, in addition to neurocognitive deficits and endocrinopathies79. vasculopathy is also termed moyamoya disease80. It is a crucial complication of radiotherapy in OPG patients, which is due to the proximity of the therapy to the circle of wills, and the modern techniques of radiotherapy, even proton therapy, cannot eliminate the risk of vasculopathy81,82.
4.4. Chemotherapy
since 2000, chemotherapy has been chosen as the first-line treatment for all ages of cancer patients to avoid radiotherapy side effects22. chemotherapy management begins with the detection of declined visual acuity. however, waiting for the patients to observe changes in their visual acuity may result in more threatening visual acuity issues. therefore, the presence of optic disc pallor throughout the management period may indicate worsening the visual acuity in patients83.
a number of regimens are used to control the progression of cancers, and the most common effective regimen is carboplatin/vincristine combination therapy, and it can be considered first-line treatment for non-surgical OPG associated with NF-184. the carboplatin regimen is well tolerated and not linked with major toxicity. however, a number of side effects may be observed such as neutropenia, allergic reactions, and thrombocytopenia85,86. temozolomide is another effective regimen, but in comparison with vineristin/carboplatin combination may not show the same advantages and effectiveness12. Falsini et al87 conducted a randomized trial in 13 patients with OPG associated with NF-1 syndrome who received nerve growth factor (NGF) eye drops after the end of chemotherapy. their results show improvement in the visual field and electrophysiological parameters.
The following is treatment recommendation algorithms for each age group5:
- Children under the age of 1 year: instantaneous chemotherapy should be considered in nearly all cases. surgery to be discussed individually depending on clinical presentation (presence/absence of hydrocephalus). no observation duration and radiotherapy.
- Children 1-5 years old: observation in the case of a small cancer size without visual impairment. about the bulky tumor, a biopsy is suggested, debulking cases to be discussed individually. whether surgery/biopsy is achieved or not, chemotherapy is to be considered if there is proof of radiological progression or visual worsening.
- Children 6-10 years old: observation in the case of a small cancer size without visual impairment. about the bulky tumor, a biopsy is suggested, debulking cases to be discussed individually. chemotherapy be considered after a duration of observation if there is proof of radiological progression or visual deterioration. conformal radiation therapy is a replacement for chemotherapy in non-nf1 patients.
- Children > 10 years old: observation in the case of a small cancer size without visual impairment. about the bulky tumor, a biopsy is suggested, debulking cases to be discussed individually. Chemotherapy or conformal radiation treatment to be considered if there is proof of radiological progression or visual worsening.
For Surgical Approach (Video) :