Article topic: Intraventricular Central Neurocytoma
Author: Abdallah Arabyat
Editor: Rahmeh Adel
Reviewer: Ethar Hazaimeh
keywords: Central neurocytoma, Histopathology, Management
Overview
Central neurocytoma is a rare intraventricular brain tumor, that typically appears in young adults with elevated intracranial pressure as a result of obstructive hydrocephalus.
The Ventricular System
The lateral, third, and fourth ventricles make up the ventricular system of the brain. These are just little cavities in the brain that are connected by tiny ducts and canals (as shown in Figure 1). The choroid plexus, which is found in each lateral ventricle and resembles a tuft, is primarily responsible for producing cerebrospinal fluid.1

Figure (1), The ventricular system of the brain 2
Each hemisphere has a pair of lateral ventricles. Each one is a C-shaped cavity that can be separated into an inferior horn that extends into the temporal lobe and anterior, posterior, and inferior horns that are each located in the frontal, occipital, and parietal lobes, respectively. The intraventricular foramen, also known as the foramen of Monro, connects the lateral ventricle to the third ventricle. On its medial side, the lateral ventricle’s choroid plexus extends into the hollow.1
Between the thalami, there is a small slit which is the third ventricle. Additionally, it has a connection to the fourth ventricle via the cerebral aqueduct or the aqueduct of Sylvius. The choroid plexuses are found above the roof of the ventricle.1
The fourth ventricle is located behind the pons and the upper half of the medulla and anterior to the cerebellum. It is continuous superiorly with the cerebral aqueduct and the central canal below. The fourth ventricle has a floor, two lateral walls, and a tent-shaped ceiling. The two lateral foramina of Luschkea and the median foramen of Magendie are the fourth ventricle’s three tiny apertures. The cerebrospinal fluid enters the subarachnoid area through these holes.1
The fourth ventricle’s choroid plexus features a T-shape. The cerebrospinal fluid is transported by the ventricular system (as shown in Figures 1 & 2). Cerebrospinal fluid appears to be actively secreted by the choroid plexuses of the ventricles, while part of the fluid may come from tissue fluid generated in the brain matter.1
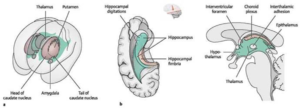
Figure (2), the ventricular system of the brain 2
Disease Entity
The lateral ventricles of the central nervous system (CNS) are the major location of Central Neurocytoma (CN), a benign primary neuronal tumor. Less than 1% of brain tumors are CN, which primarily affects young adults. 3 CN is a grade II brain tumor, according to the World Health Organization (WHO) classification. Though its cause is uncertain, central neurocytoma is thought to develop from subependymal cells of the lateral ventricles and neuronal cells of the septum pellucidum.4
Epidemiology
CN was first reported by Hassoun et al. in 1982, and it is still a rare diagnosis. Small series and lone case reports account for a large portion of the information we have regarding CN. Asian people have reported more incidences of CN than Caucasian ones, with higher rates in Korea, India, and Japan.6 CN tends to affect both men and women equally, primarily affecting individuals in their second or third decade of life. 7
Pathology
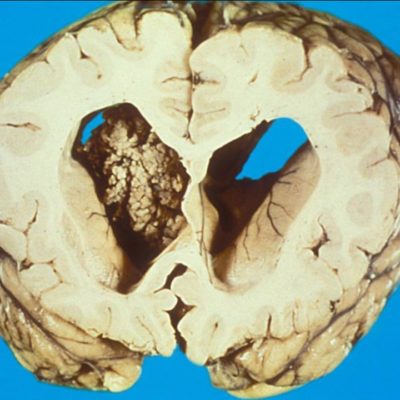
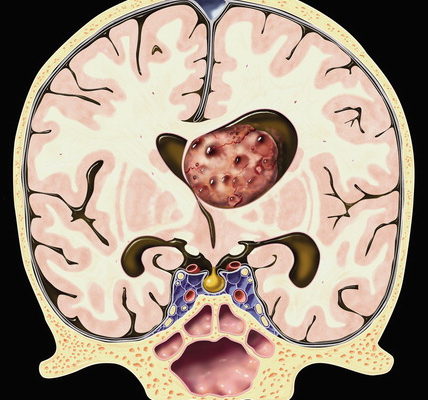
A homogenous population of spherical cells with little cytoplasm in a fibrillary backdrop make up central neurocytomas. The nuclei have punctate nucleoli and finely specked chromatin. There is often a fragile capillary vascular network (as shown in figure 3).8 The tumor cells are immunoreactive for synaptophysin, NSE, and other neural markers and exhibit ultrastructural signs of neural development. Rare atypical forms with enhanced mitotic activity, necrosis, and cytologic atypia have been observed. Ependymomas and intraventricular oligodendrogliomas are frequently included in the differential diagnosis. 8
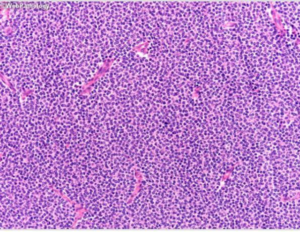
Figure 3, A uniform population of round cells with scant cytoplasm in a fibrillary background. 8
Synaptophysin, a transmembrane glycoprotein present in presynaptic neuronal vesicles, is the most trustworthy diagnostic marker.9
Western blotting or immunohistochemistry is used to measure synaptophysin reactivity. However, it’s important to remember that the lack of synaptophysin does not rule out the diagnosis of CN. According to studies, it is possible to distinguish between CN and other CNS neoplasms using an immunohistochemical panel that includes the Epithelial Membrane Antigen (EMA), vimentin, and neuronal nuclear protein (NeuN). When EMA and vimentin are negative and NeuN is positive, CN is more likely to be diagnosed than ependymoma or oligodendroglioma.3
Imaging Features
General
Intraventricular mass in the supratentorial region that appears cystic or bubbly. Near the foramen of Monro, it is frequently found with a lateral ventral frontal horn or body attached to the septum pellucidum or lateral ventricular wall, either with or without third ventricular extension. It often displays calcification.
CT scan
Heterogeneous density intraventricular mass due to solid and cystic components with and without calcification.
MRI
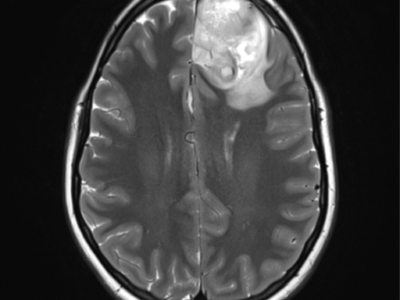
T1: heterogeneous but predominantly isointense. Figure (4)
T2: usually hyperintense with cystic or bubbly appearance; ±flow voids. Figure (5)
FLAIR: heterogeneously hyperintense. Figure (6)
T2*/GRE/SWI: black signal blooming in foci of calcification
T1WI+C: heterogeneous enhancement. Figure (7)
MRS: increased Cho, decreased NAA; glycine peak at 3.55
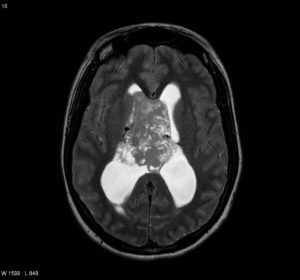
Figure (4) Axial T1
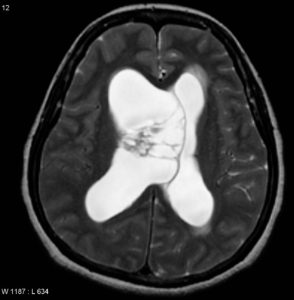
Figure (5) Axial T2
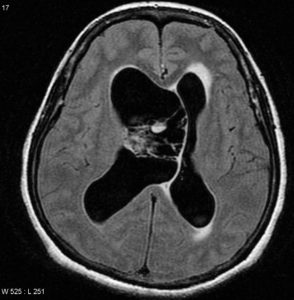
Figure (6) Axial FLAIR
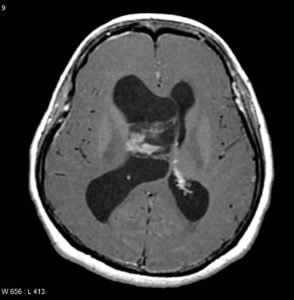
Figure (7) Axial T1 C+
Previous Figures are selected images from MRI of the brain including post-contrast sequences that demonstrate a large intraventricular mass filling and expanding the body of the right lateral ventricle. It has a high T2 signal, and wispy regions of intermediate T1 signal giving it a bubbly appearance. Following contrast administration only minimal heterogeneous enhancement is present.10
Clinical presentations
Due to the interventricular foramen obstruction, patients may have elevated intracranial pressure and hydrocephalus.17
Headaches, seizures, nausea, vomiting, and changes in memory or vision are just a few of the possible side effects that patients may face.18,19
Treatment
Institutional case studies provide a guide for managing CN. The standard of care for treating CNs is gross total resection (GTR) surgery, which is successful in 30 to 50% of cases and has a 5-year survival rate of 99% according to studies.5 Patients with subtotal resection (STR) have a higher chance of recurrence and are given radiosurgery or adjuvant radiotherapy as treatment. Studies have been inconclusive as to whether adjuvant fractionated radiotherapy or adjuvant stereotactic radiosurgery is better for local tumor control, but both have proved to be better when GTR cannot be achieved.9
Prognosis
CNs often have low rates of local recurrence and are benign tumors amenable to surgical resection. According to specific research, the five-year survival rate for CN following GTR can reach 99%, but the five-year survival rate for CN merely reaching STR is 86%. 9