Article topic: Neural Tube Defects
Author: Rania Sanouri
Scientific editor : Dr.Omar Jbara
Linguistic editor: Philip Sweidan
Overview
Neural tube defects (NTDs) are birth anomalies attributed to a problem in or failure of closure of the neural tube during embryogenesis. The level of failed closure decides the type and the severity of the neural tube defect. Usually, NTDs are believed to be multifactorial, mainly due to genetic and environmental causes. NTDs are common with a prevalence from 0.5 to 10 per 1,000 pregnancies (1).
Epidemiology and risk factors
Since many cases of NTDs end up as spontaneous abortions or legal terminations, data about prevalence is affected (1). Many cases are non-syndromic, other NTDs are due to chromosomal aneuploidy including trisomy 13 & trisomy 18, which account for less than 10%. The prevalence of NTDs in miscarriage & stillbirths is higher than in term pregnancies. It also has ethnic and regional variations, for instance, the incidence of NTDs in Mexico is 3.2 in 2000 births, which is higher than in the United States. Moreover, regions in China have 20 times higher prevalence than in the US(2).
Regarding genetic predisposition, a history of pregnancy with an NTD-affected fetus will have a recurrence risk of 3% in the subsequent pregnancy and 10% if after conceiving a second NTD embryo. In twins, the NTDs’ concordance rates among monozygotic twins are7.7%, which is higher than the rate for dizygotic twins (4.4%). For some types of NTDs, there is gender predominance; however, no final evidence has been identified (2).
Many genetic and non-genetic factors are involved in the abnormal closure of the neural tube. One hypothesis called “multifactorial threshold model” assumes that only in synergism can these factors cause failure of closing the neural tube and production of abnormal phenotype. This excludes the ability of one factor to produce NTD and cause disruption to embryogenesis (2).
Non-genetic factors include those from the environment ex: air pollution, maternal factors, diseases, nutrition, exposure to occupational chemicals or physical agents and substance abuse (2).
Classification
According to Lemire classification, neural tube defects are classified into two main defects: neurulation & post-neurulation defects. Neurulation defects include those that occur due to failed closure of the neural tube and thus are open lesions. Examples: Craniorachischisis, anencephaly AKA “exencephaly” and meningomyelocele (myelomeningocele (MM) and myelocele). Whereas post-neurulation defects result in closed, skin-covered lesions sometimes referred to as migration abnormalities, which could be cranial or spinal defects (3).
Post-neurulation cranial defects include microcephaly, hydranencephaly (loss of most of cerebral hemispheres), holoprosencephaly, lissencephaly, porencephaly, agenesis of corpus callosum, cerebellar hypoplasia “Dandy-Walker syndrome” and megalencephaly (3). Post-neurulation spinal defects include: diastematomyelia, diplomyelia and syringomyelia(3).
Embryonic Basis of NTDs: Neural Tube Closure
The knowledge about principles of neural tube defects is taken from experimental models mainly mammals and amphibians because it is difficult to get access to neurulation staging from human embryos (1).The neural tube closure process is complex and involves many events occurring at the cellular level such as convergent extension, apical constriction and interkinetic nuclear migration. In addition, there are many molecular processes including the non-canonical Wnt/planar cell polarity pathway, Shh/BMP signalling and Grhl2/3, Pax3, Cdx2 and Zic2 transcription factors(4).The primary neural tube closure is generally the result of folding and fusion of the neuroepithelium(1). Other origins like non-neural ectoderm (NNE), mesoderm and notochord have been implicated in regulating the neural tubeclosure (NTC). Any failed changes of neurulation morphology will lead to disturbances in NTC, generating neural tube defects which are among the commonest human birth defects. Understanding of the mechanisms of NTC may assist in the development of methods for predicting and preventing NTDs (4).
Neurulation
Neurulation, which occurs at the 3rd week of embryonic life, is the first step in neural tube development. It involves thickening of the ectoderm from node of Hensen caudally reaching the prochordal plate rostrally. Studies done on xenopus embryos, concluded that ectoderm is made by default to have a neural fate that is usually inhibited by BMPs (Bone morphogenetic proteins). However, in higher vertebrates, this process is more complex and involves other factors that play a role in inhibition like: FGF (fibroblast growth factors), β‐catenin and possibly calcium transient(5).
Neurulation has two subtypes related to the stages of closure:
- Primary Neurulation:
Neural tube formation mainly involves bending of the neuroepithelium at the midline to generate two neural folds that will elevate and fuse (primary neurulation) (1).The formation of the neural groove in the midline results from the different rates of cell proliferation and changes in cell shape and movement in the neural plate. Cell‐shaping processes including (apicobasal elongation, apical narrowing, and basal expansion), cell movement and adhesion, which are superimposed by the forces from mesenchyme and the surrounding surface ectoderm, will elevate and converge the neural folds along the dorsal midline and fuse with each other to form the neural tube. Surface ectoderm at the edges of the neural folds will fuse as well. Neural crest‐derived ectomesenchyme in the cranial region and in the trunk will later spread around the neural tube under the surface ectoderm and becomes the primordia of the meninges, axial skeleton, and muscles (5).
Neural tube closure is discontinuous with distinct sites of initiation located at characteristic axial levels in both humans and mice. Requirements for closure depend on certain morphological and molecular basis, and differ along the body axis. Therefore, NTDs occur due to failure of initiation points or disruption of progression of closure between these sites (Figure 1)(1).
Closure of the NT first begins on day 8 at the level of the hindbrain/cervical region during embryological life of mice (closure 1) (Figure 1a), and failure of this event usually leads to craniorachischisis. The next day, closure 2 usually takes place at the caudal forebrain. Once contact and fusion are established between the tips of the neural folds, closure spreads bidirectionally from the sites of closures 1 and 2 in a caudal direction from the rostral end of the neural tube (closure 3). The neuropores (open regions of neural folds) gradually will contract to complete the closure process of both the anterior neuropore (between closures 2 and 3) on embryonic day 9 and the hindbrain neuropore (between closures 1 and 2) later. In humans, however, Cclosure 1 occurs at 21 days of fertilization and the whole process of closure completes by 28 days post fertilization (1).
Cranial neural tube defects (like anencephaly) result from failure of closure 2, or incomplete fusion between closures 1 and 2, which closes the midbrain and the hindbrain. If progression from the anterior end of the neural plate (closure 3) fails, this causes a split face with forebrain anencephaly (1).
Neurulation of the spinal part of the NT progresses caudally while the embryo continues to grow. Primary neurulation completes with final closure of the posterior neuropore on day 10. If the progression of closure fails, a persistent opening of the posterior neuropore results in spina bifida, and the size of the lesion directly correlates to the axial level at which closure stops(1).
- Secondary Neurulation
Secondary neurulation is the formation of the NT in the lower regions of sacrum and coccyges without neural fold closure (4). This neurulation process is mainly about condensation of specific type of cells known as tail-bud derived cells that form a type of epithelium which closes the neural tube(2). Disruptions in this transitional process will mostly cause closed or skin-covered lesions associated with tethering of the cord and ectopic lipomas (Figure 1).
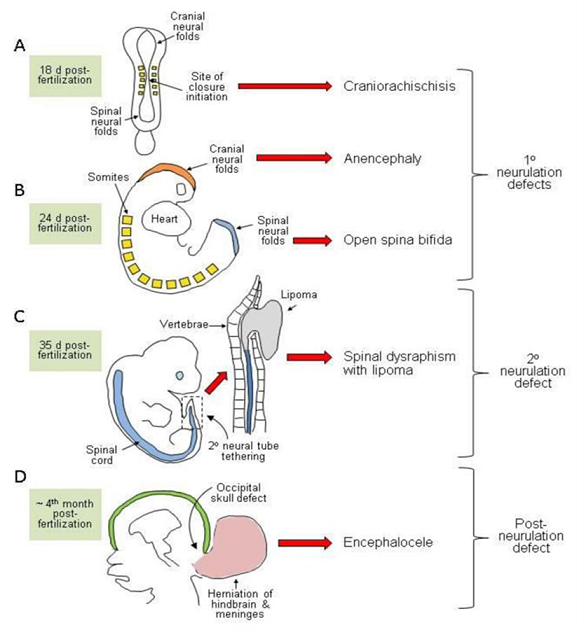
Figure 1: Diagrammatic representation of the developmental origin of malformations broadly classified as neural tube defects in humans (1)
Etiology & Pathogenesis
Development of the neural tube is a multi‐step process controlled by genetic and environmental factors. It involves gene–gene, gene–environment and gene–nutrient interactions. Despite many years of research, the exact cause of NTD remains not fully understood. Nowadays, it is largely agreed that most NTD cases are of multifactorial origin, having a significant genetic component to their etiology that interacts with several environmental risk factors (1).
Genetic
Generally, genetics of NTDs is still not fully understood & very complicated. Single gene disorders like: Waardenburg syndrome, Fraser syndrome & Meckel-Gruber syndrome were found to be associated with NTDs. Moreover, chromosomal anomalies including autosomal trisomy have higher incidence of spina bifida. Other risk factors include: monozygotic twinning and consanguinity (5).
Environmental
Multitude of environmental risk factors are related to congenital neural defects including physical agents such as radiation, hyperthermia and stress, as well as drugs (e.g. thalidomide, folate antagonists and antiepileptics and hypervitaminosis A), alcohol, organic mercury, lead, maternal infections (e.g. rubella, syphilis, cytomegalovirus, Toxoplasma gondii), and maternal metabolic conditions (e.g. phenylketonuria, diabetes mellitus). Exposure to noxious agents such as organic solvents, anesthetics, pesticides and paints has been reported to be related to an increased risk of NTD (5).
Bad maternal health status has been attributed to enhance the risk for NTD in the offspring. The antenatal screening study should include women with spontaneous pregnancy loss, known to be associated with a high incidence of NTD. It is important to point out that contrary to frequent claims, CVS anomalies (not NTDs) are more common in infants of diabetic mothers (5).
Nutritional factors and Folate
Folate one-carbon metabolism (Figure 2) comprises complex reactions that mediate transfer of one-carbon groups for several biosynthetic processes. It is required for nucleotide biosynthesis and methylation reactions that occur in neural tube closure. Abnormal thymidylate and purine biosynthesis have been identified in mouse NTD models and some NTD cases. Deficient methylation may also be implicated in NTDs (1).
Given that lower socioeconomic status and increased risk of birth defects are linked, there is possibility that nutritional factors are also related to NTDs. It was found that mothers of NTD fetuses had lower levels of vitamin B9 (folate) in their serum, necessitating trials of folic acid-containing supplements to prevent NTD recurrence. A multi-center randomized controlled trial confirmed that maternal folic acid supplementation (at 4 mg per day) significantly reduces the recurrence risk, and other clinical trials proved evidence for reduction of occurrence risk as well (1).
Data have shown an inverse relationship between blood folate concentration and risk of an affected pregnancy. Folate levels below the normal range may contribute to NTD development in genetically susceptible individuals. For example: gene- environment interaction was studied in mice to show that folate deficiency does not cause NTDs unless combined with a mutation of a predisposing gene, such as Pax3 gene (1).
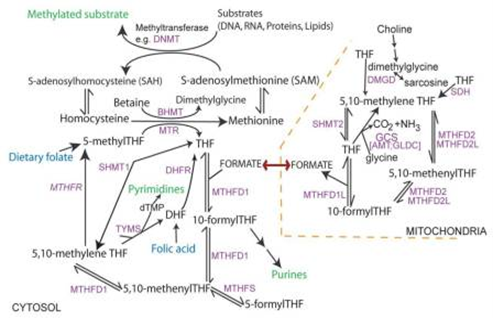
Figure 2: Overview of folate one-carbon metabolism (1)
Pathogenetic Mechanisms of NTD
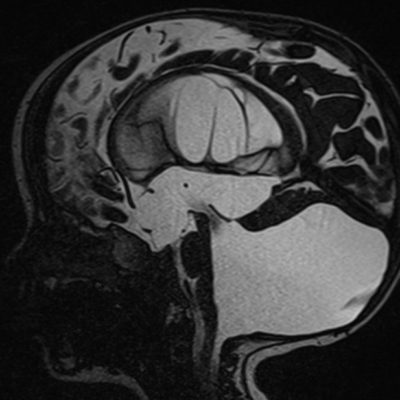
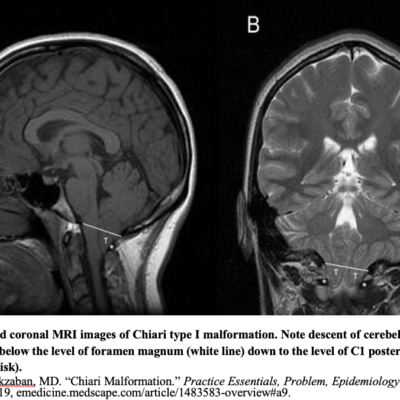
NTD are often thought to result only from a primary failure of the neural tube closure. however, there is now clinical and experimental explanation to support secondary reopening of an already closed NT, causing NTD. Human embryo studies indicate that post‐closure lesions are late in onset. NTD affect not only regions of the neural tube but other non‐neural organs as well. Myelomeningoceles are virtually always associated with Chiari II malformations (Figure 3) (5). The developmental interruption of neurulation NTD with other anomalies more than isolated NTD(5).
A study done by Seller and Kalousekin 1986 compared the frequency and pattern of isolated NTD and those of NTD associated with other abnormalities. They note that significant bunch of developmental defects are associated with total craniorachischisis and upper thoracic spina bifida, less frequently with anencephaly and lumbosacral spina bifida and never with sacral spina bifida(5).
This definitive pattern possibly implies a connection between the mechanisms by which NTD and associated anomalies develop(5).

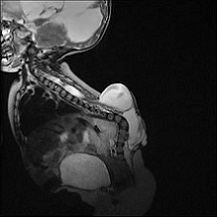
Figure 3: Myelomeningocele in Chiari II malformation, Case courtesy of Dr Arthur Daire (8)
Clinical Presentation & Complications
In general, NTDs share very similar pathogenic features even though they originate differently. Open NTDs result from failure of closure at the site of initiation or incomplete closure after initiation was successful (Figure 1). NTDs can be recognized during or immediately after neurulation because neural folds will be kept open. However, at later stages, the morphological appearance changes considerably due to degeneration. In cranial NTDs, opened neural folds bulge from the developing brain, known as exencephaly. In anencephaly, the skull vault fails to form, leaving the exposed neural tissue liable for degeneration. Both anencephaly and craniorachischisis are lethal at time of birth or shortly after(1).
In the spinal region, opening in the neural folds prevents the sclerotome-derived vertebral arches from covering the neuroepithelium, leaving an opening in the vertebral column known as spina bifida. The neural tissues may be found inside their meningeal sac and can extrude through the opened vertebrae (myelomeningocele; spina bifida cystica) or directly to the amniotic fluid known as myelocele. Babies having with spina bifida need the proper medical care but will suffer neurological impairment and other conditions including hydrocephalus, vertebral abnormalities as well as genitourinary and gastrointestinal disorders (1).
Diagnosis and Maternal-Fetal Surgery
NTDs can be discovered during early prenatal period by ultrasound. Postnatal management for babies born with open spina bifida usually requires surgery to close and cover the lesion, in addition to other following surgeries to alleviate tethering of the spinal cord, treat hydrocephalus, and/or deal with orthopedic and urological problems.
New techniques to provide in-utero fetal surgery for spina bifida aim to limit the possible secondary damage that may result due to exposure of nervous tissue to the surrounding amniotic fluid, and also to improve overall neurological outcomes compared to conventional postnatal repair (1).
Disorders of the Closed Neural Tube
Other conditions which include anomalies of the closed brain or spinal cord and are considered as NTDs (Figure 1) involve other less common group of closed spinal NTDs where vertebral arches are malformed but still covered by skin. Examples include spina bifida occulta and spinal dysraphism. The more severe subtypes are associated with various abnormalities of the spinal cordlike lipomas (Figure 1c), and anorectal abnormalities. The embryonic origin of closed spina bifida is presumed to have abnormalities of secondary neurulation (1).
In encephalocele (Figure 1d), abnormal development of the skull allows herniation of the closed neural tube through the affected bony region, the same applies to meningocele (a closed spina bifida in which the herniated defect consists of meninges extruding through the bony vertebra) (1).
Prevention
NTDs are known to have effective preventive methods available nowadays. Studies have shown the relation and importance of vitamin intake during pregnancy with respect to NTD outcome. One study conducted by the UK Medical council proved that daily 4 mg folic acid supplementation can reduce NTD risk by three times. In addition to many other non-randomized trials, this concludes that folic acid supplementation during pregnancy in a dose of 0.4–5 mg per day can prevent NTD births. Molecular mechanisms & cellular reactions by how folate prevent NTDs are still not completely understood. Maternal folic acid peri-conceptional supplementation strategies have been implemented. However, epidemiological studies have shown that not all NTDs can be prevented and a sub-group of folic acid-resistant NTDs still occur. Experiments done on animals support inositol supplementation in decreasing the recurrence of NTDs, and it can be used for non-folate responsive NTDs (2).
Data from a trial of pre-conceptional vitamin supplementation for the prevention of neural tube defects suggested that the risk of recurrence of neural tube defect is influenced by the number of previous neural tube defects, area of residency, immediately prior miscarriage, and the inter-pregnancy interval. Also, the study concluded that vitamin supplementation was behind the considerable difference in rates of NTDs between supplemented and non-supplemented mothers (6).
Diagnosis & Treatment
Prenatal detection of neural tube defects:
Generally, two techniques are used for screening: biochemical testing of maternal blood for alpha-fetoprotein (AFP) and the use of 2D ultrasound (7).
Alpha fetoprotein( AFP):
Screening of the maternal serum AFP, a glycoprotein secreted by the fetal liver and yolk sac, is usually reserved for open NTDs. However, increased AFP is not diagnostic for open NTD, as other anomalies (omphalocele, gastroschisis and congenital nephrosis can have raised AFP levels as well) (7).
High maternal serum AFP between 15 & 20 weeks of gestation carries a high relative risk for NTDs; 34% of all major congenital defects are linked to abnormal values of AFP. In spina bifida, the sensitivity of maternal AFP is 91%. Overestimation of the gestational age may misinterpret an elevated AFP as normal or a normal AFP as elevated in underestimated gestational age. Closed lumbosacral spinal defects (20% of spina bifida patients), may be missed by AFP screening, and on ultrasound as well (3).
Ultrasound
The significance of ultrasonography is that it can estimate the gestational age accurately, detect around 95% of spina bifida cases prenatally, and discriminate NTDs from non-neurological causes of increased AFP (e.g., omphalocele) (3). Rates of NTDs discovered by ultrasound are affected by the gestational age and the type of NTD. Studies done during first trimester can detect up to 90% cases of anencephaly and 80% of encephalocele. However, spina bifida is best detected during the second trimester (around 95% of cases). An important part of screening is to look for any additional malformations that may accompany NTD (7). Imaging techniques for diagnosing NTDs include:
2D ultrasound
Anencephaly
Anencephaly, or exencephaly, (Figure 4) should be diagnosed after 12 weeks of gestation, as the skull vault would be clearly visualised. Ultrasound findings at the 1st trimester include:
- Absent head contour and exposed neural tissue “lobulated appearance”.
- Decreased crown-rump length.
- Absent calvarium. (Figure 5)
Absence of the skull and neural tissue will cause disrupted fetal swallow and results in polyhydramnios detected at the 2nd or 3rd trimester (7).
Cephalocele
Cephaloceles are mostly visualized as herniated meninges (meningocele) or brain tissue (encephalocele) through a skull defect. Cephaloceles are usually associated with microcephaly, Dandy-walker malformation, ventriculomegaly, cardiac defects and facial clefts; 75% of cephaloceles herniate in the occipital bone (7).
- Spina bifida
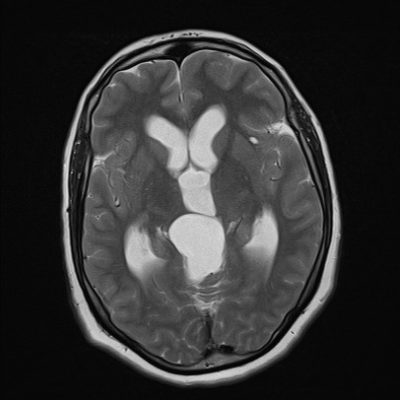
The detection rate of open spina bifida cases usually reaches up to 100%, as they can be easily recognized by two main signs: the “lemon” and the “banana” signs. Lemon sign reflects the skull shape in transverse plane. It can be seen as early as 13 weeks of gestation, and is present in almost all cases of between 16 and 24 weeks of gestation (Figure 6). However, the lemon sign is not to specific for NTDs, as around 1% of normal fetuses can have a similar concavity (7).
The banana sign describes the shape and reflects the traction of the cerebellum, correlated to the leakage of spinal fluid from the open spinal lesion. Fetal MRI can also show spinal/vertebral dysraphism with fluid-filled sac of meninges extruding at the back.
3D ultrasound
3D ultrasound gives an exceptional view of spinal lesions for diagnosing NTDs. One review article summed up the significance of 3D ultrasound as follows:
- Complete 3D visualization of the spine and other structures from other planes.
- Data from ultrasound can be reviewed after the patient has left the room.
- Provides multitude of algorithms for observing different features of the same structure.
- Multiple planes can be displayed at the same time.
- Ability to transmit data over networks for consultation in other centers.
- The ability to use software for interactive educational tools(7).
Amniocentesis
In cases of pregnant ladies with history of myelomeningocele, prenatal amniocentesis is recommended to exclude spinal anomalies not seen by ultrasound, and to provide the best postpartum care in case of MM diagnosis. Increased AFP in the amniotic fluid peaks around 15 weeks of pregnancy in cases of open NTDs. Amniocentesis is a risky procedure carrying 6% chance of fetal loss(3).
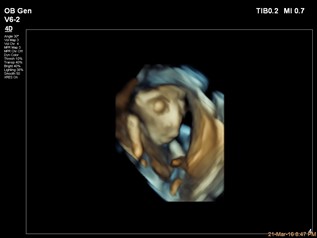
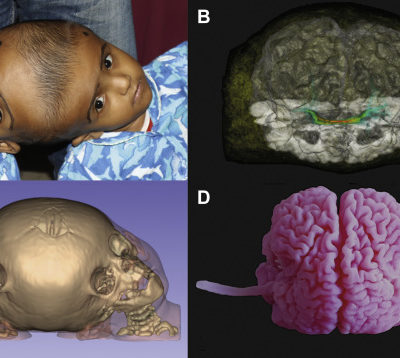
Figure 4: 3D image of anencephaly, Case courtesy of Dr Islam Mohamed El Gezeiry(9)
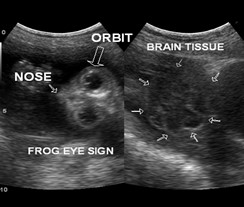
Figure 5: Abscense of bony calvarium, Case courtesy of Dr Mohd Imran (10)
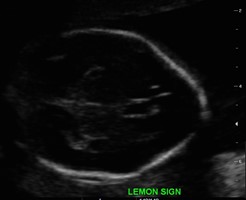
Figure 6: Lemon sign,Case courtesy of Dr Praveen Jha (11)
Diagnosis & Prognosis
- Craniorachischisis
A disorganized mass of cerebral tissue with cervical hyperextension may be observed by ultrasound. Other differential diagnoses for craniorachischisis include nuchal tumors (e.g.,lymphangiomas), Klippel–Feil syndrome and Jarcho–Levin syndrome, also known as “spondylocostal dysostosis” (2).
Craniorachischisis is a fatal condition with no cure or surgical management and usually death of newborns is inevitable (2).
- Anencephaly
It is very crucial to distinguish between anencephaly mentioned above and amniotic band-related head destruction, also characterized by lost cranial vault, as anencephaly tends to reoccur in subsequent pregnancies unlike the other sporadic entity (2).Anencephalic infants often have no other malformations, plus the cranial defect is symmetrical in contrast to that in the cases of amniotic bands syndrome.
Anencephaly is another lethal condition commonly detected during early gestation and legally terminated. However, minority of cases that progress to term die within few days after birth (2).
- Myelomeningocele (MM)
Fetal MM repair can reverse the cerebellar displacement. Clubfoot (abnormal internal rotation of the feet) is nearly presented in all cases of MM as a result of prolonged decrease in leg movements during pregnancy (2).
Prognosis: Myelomeningocele or myelocele cases have good survival if they were not associated with other serious defects. The neurological impairment and clinical deficits correlate with the level of the lesion, those usually include: Bladder dysfunction, fecal incontinence, and sexual dysfunction (2).
Results of management of MM Study trial (MOMS) declared that in utero surgical intervention before 26 weeks of gestation can conserve neurologic function in some cases. However, prenatal surgery is very risky and can cause prenatal delivery or premature rupture of membranes (PROM).Cases that come with hydrocephalus can lead to intellectual disability, still it is quite uncommon (25%) (2).
MM surgical repair should be started within the first 72 hours after birth. Cardiopulmonary and genitourinary systems assessments should be undertaken, and a cranial ultrasonography should be performed later (to check the size of ventricular system). Any delay after that can increase morbidity & mortality. If closure of a MM was delayed after 72 hours, cultures from the neural placode should be taken to exclude any infection. If cultures return positive, an external ventricular drain should be placed, and appropriate antibiotics administered until the infection clears (12).
- Encephaloceles
Encephaloceles have an annual incidence of 1 in 10,000 born babies in the US. The cystic mass protruding through the defect can be detected by ultrasound. Encephaloceles can be in any location of the cranial vault (anterior, parietal, or occipital). The occipital is the most common location and can be seen in Chiari II malformation. however, Chiari II malformation can have protrusion from the 1st cervical vertebra or herniation of the cerebellum with hydrocephalus. Other anomalies include: Microcephaly & ventriculomegaly (2).
Differential diagnosis for encephalocele may include craniofacial masses including: (hemangiomas, nasolacrimal duct cysts, neuralgia heterotopia, lymphangiomas and lipomas (2).
Overall, the prognosis varies depending on the content of the mass and its location. The more the mass is located rostrally, the better the outcome. Other factors that play a role in the prognosis include hydrocephalus and the degree of brain involvement. Traction of some parts of the brain caused by the herniation can lead to different levels of intellectual disabilities, epilepsy and motor and sensory impairment. The management of encephaloceles is only surgical repair (2).
- Spina bifida Occulta
Spina bifida occulta are covered defects that usually involve the lower lumbar and sacral regions and result due to incomplete vertebral arches (2).
Gross dysmorphology shows cutaneous marks like: Nevi, subcutaneous lipomas, hair tufts, hemangiomas, depigmentation, and dermal sinus tract. These could be the only signs of a spina bifida occulta. Spina bifida occulta can come with other skeletal defects such as scoliosis, asymmetrical gluteal cleft, and leg length discrepancy (2).
The prenatal diagnosis is very challenging, since its not associated with secondary cranial anomalies. The most common detectable form in utero is the closed spina bifida associated with intradural lipoma. Lipomas can appear very similar to meningoceles on ultrasound (2).
High resolution ultrasound can be used before ossification of the vertebral body takes place (before 6th months), yet its sensitivity is still low, especially in cases of a subcutaneous mass (2). The more preferred diagnostic tool is magnetic resonance, which usually requires sedation and can be costly (2).
Spina bifida occulta is a less severe spectrum of spinal dysraphism often detected later in life when the neurological symptoms begin to show up due to cord traction because spinal cord growth used to be much slower than the spinal column during intrauterine life. However, only after delivery can the distal part of the spinal cord reach its normal level and become more liable for injury. In cases of thickening of filum terminale, increased physical activity or lumbar trauma, the presentation may be earlier in childhood. Symptomatic patients with neurological progression will require neurosurgical treatment.
- Meningocele
Macroscopically resembles myelomeningocele, thus can be difficult to distinguish between them by ultrasound prenatally. Through the location of a neural placode, however, we can tell the difference macroscopically (2). Neural placodes are focal thickenings giving rise to various cells. For example, cranial neural placodes (thickenings in the head of an embryo) can give rise to neurons of a cranial nerve sensory ganglia and other sensory organs(12).
When the placode is out of the the canal and through the vertebra, and because there is expansion of the subarachnoid space, this will create a defect to the cutaneous tissue, and results in a myelomeningocele. On the other hand, the subarachnoid space is not expanding in a case of a myelocele, plus the placode is kept in or deep to the cutaneous surface (2).
The herniated mass is often covered by skin with different degrees of dysplasia, also the lesion is usually pedunculated and easily compressed with transillumination (2).
Prenatal diagnosis by ultrasound where spinal dysraphism may be seen with an associated cystic mass. The characteristics of the cystic mass are comparable with that of the myelomeningocele, although they lack septa in the herniated mass, and the cranial anatomy is unremarkable (2).
Meningocele cases usually have no lower limb deformities or sphincter dysfunction with preserved neurological function on exam. The treatment of meningocele involves surgical correction (2).