
ِArticle Topic: Highly Aggressive Multiple Sclerosis
Author: Abdeljaber Tayyem
Editor: Haneen Al-Abdallat, Haneen A. Banihani
Reviewer: Lobana Mahdawi
Keywords: Aggressive Multiple Sclerosis, Expanded Disability Status Scale, Humanized Monoclonal Antibodies, Immunosuppressants, Induction therapy, Escalation therapy
Overview
Multiple sclerosis(MS) is a chronic inflammatory demyelinating disease that affects the central nervous system (CNS). It has various clinical presentations according to the site of the central nervous system involved and is categorized according to clinical course into clinically isolated syndrome, relapsing-remitting, secondary progressive, and primary progressive according to 2013 disease course definitions (1). A subset of MS patients experience an aggressive form of the disease where they progress rapidly to a disability or have a higher number of relapses. They need special attention to define this form of the disease to facilitate early identification and intervention to prevent rapid progression to disability (2)(3).
Etiology and pathogenesis
Generally speaking, MS is thought to be caused by the accumulation of genetic and environmental risk factors, which lead to the activation of autoreactive lymphocytes in the CNS, including B lymphocytes which release proinflammatory cytokines, chemokines, and autoantibodies, as well as T lymphocytes which include CD8+ T cells that release perforins and granzymes which will damage the axons and found in vast areas of brain parenchyma as well as CD4+ T cells which are more concentrated in the perivascular cuffs. In addition to glutamatergic excitotoxicity, this inflammatory cascade, hypoxia, and reactive oxygen species lead to calcium influx, thus causing mitochondrial dysfunction, oxidative stress, and cell death, which will cause demyelination and axonal damage. However, aging-related neurodegeneration, meningeal lymphoid-like follicles, and innate immunity involving microglial activation, which is not targeted by current MS therapies, are thought to be the cause of late progression of the disease where there is increased neurodegeneration, axonal damage, and a more indolent form of inflammation are involved. (4)(5)(6)
The development of permanent disability in MS is related to axonal loss, i.e., a higher rate of axonal loss would relate to a more aggressive disease. Macrophage infiltration contributes to inflammation, and decreased myelin trophic factors increase axons’ vulnerability to inflammation, leading to axonal transection and cortical involvement and causing neuronal death. This leads to a more aggressive and less treatment-responsive form of MS. There is also an association between dysregulated subgroups of T cells and NK cells and decreased diversity of T cell receptors on CD4+ T cells with aggressive MS (2).
Risk factors
Multiple risk factors were associated with aggressive MS, such as Male sex, older age at onset(more than 35), smoking, and non-white ethnicities. Multiple genetic markers are also related to aggressive MS including HLA-DRB1, APOE4, PD-1, MGAT-5, and BDNF. (2)(3)(6)
Clinical presentation
Optic neuritis
Patients present with eye pain and visual blurring or vision loss for hours to days. In a fundoscopic examination, the optic disc appears normal (retrobulbar optic neuritis) in two-thirds of cases or swollen (papillitis) in one-third of patients. Relative afferent pupillary defect (Marcus Gunn pupil) can also be present. (7)
Myelitis
This could be either transverse myelitis, in which sensory, motor, and bladder or bowel spinal cord tracts are involved, or partial myelitis, where one or more spinal cord tracts are involved but not all. However, MS typically presents with partial myelitis. (7)
Brainstem syndromes
Brainstem could be affected in MS by the involvement of cranial nerves (III, IV, and VI), causing diplopia, medial longitudinal fasciculus damage leading to ophthalmoplegia, cranial nerve VII leading to facial palsy, cranial nerve VIII leading to vertigo, cranial nerves (IX, X or XII) causing bulbar symptoms including dysphagia or dysarthria or cranial nerve V leading to sensory impairment in the face. However, facial sensory impairment can also be related to cervical spinal cord lesions. In addition, there might be cerebellar involvement leading to unilateral ataxia, dysdiadochokinesia, dysmetria, or cerebellopontine involvement, causing pendular nystagmus. (7)
Motor symptoms
Since the corticospinal tracts are usually involved, the upper motor neuron syndrome usually presents with hyperreflexia, spasticity, weakness, and positive Babinski sign . (7)
Sensory symptoms
Sensory symptoms include numbness, paresthesia, pain, and Lhermitte sign in which neck flexion causes an electric shock-like sensation running through the spine, indicating cervical or upper thoracic dorsal column involvement. (7)
Imbalance
It is caused by cerebellar involvement, vestibular dysfunction, or sensory impairment (sensory ataxia). (7)
Cognitive, psychological, and functional symptoms
The most common cognitive symptoms of MS are mental slowing, executive function impairment, or long-term verbal and visual memory. Less commonly, MS patients can have dementia. However, cognitive impairment indicates white matter, cortical involvement, and brain atrophy. Depression is present in some patients of MS as well as fatigue which is a highly prevalent presentation of MS and is related to depression or even diseases other than MS, warranting further investigations. (7)
Uthoff’s phenomenon
Patients experience heat sensitivity in which symptoms are exacerbated by heat due to higher temperatures causing less efficient conduction in demyelinated fibers (7).
Bladder and bowel dysfunction
Bladder involvement is manifested in a neurogenic bladder, leading to patients experiencing urinary retention, urinary frequency, difficulty initiating urine stream, or urinary incontinence. MS can also lead to bowel involvement, which is less common than bladder involvement, and usually presents as constipation and less commonly as incontinence, where a severe spinal cord injury could be related (7).
Sexual dysfunction
Erectile dysfunction is the most common symptom in men, and fatigue or loss of libido is common in women (7).
Headaches
Migraine is the most common type of headache. However, MS is not related to an increased risk of migraines, but migraines are linked to an increased risk of MS.(7)
MS exhibits multiple disease courses. These include clinically isolated syndrome, where patients have an initial attack of MS but need additional attacks to prove the diagnosis of MS, and relapsing-remitting form, in which patients suffer from multiple attacks with periods of recovery that could be partial or complete without progression of the disease during recovery periods. Such disease courses also include a secondary progressive form in which relapsing-remitting disease becomes progressive with worsening disability and is associated with relapses, as well as a primary advanced form in which the disability progresses at disease onset without early relapses or remissions. (1)
The expanded disability status scale (EDSS) can be used to define aggressive MS and predict the risk of progression to aggressive MS.(2) The EDSS scale was established in 1983 and is still used to describe the degree of disability and response to therapy in MS using multiple functional systems (Table 1). (8) Initially, EDSS was named the Disability status scale (DSS), which included the same functional systems but differed in the number of steps on the scale, which were ten steps on DSS and became 20 in EDSS (Table 2). (9)
Table 1: functional systems, reprinted from Piri Çınar B, Güven Yorgun Y(9):
| 1) Pyramidal Functions
0. Normal. 1. Abnormal signs without disability. 2. Minimal disability. 3. Mild or moderate paraparesis or hemiparesis. 4. Marked paraparesis or hemiparesis, moderate quadriparesis; or monoplegia. 5. Paraplegia, hemiplegia, or marked tetraparesis. 6. Quadriplegia. V. Unknown. |
| 2) Cerebellar Functions;
0. Normal. 1. Abnormal signs without disability. 2. Mild ataxia. 3. Moderate truncal or limb ataxia. 4. Severe ataxia, in all limbs. 5. Unable to perform coordinated movements due to ataxia. V. Unknown. X. Used throughout after each number when weakness (grade 3 or more on pyramidal) interferes with testing. |
| 3) Brain Stem Functions;
0. Normal. 1. Signs only. 2. Moderate nystagmus or other disability. 3. Severe nystagmus, marked extraocular weakness, or moderate disability of other cranial nerves. 4. Marked dysarthria or other marked disability. 5. Inability to swallow or speak. V. Unknown. |
| 4) Sensory Function (revised in 1982);
0. Normal. 1. Vibration or figure-writing decreases only in one or two limbs. 2. Mild decrease in touch or pain or position sense, and/or moderate decrease in vibration in one or two limbs; or vibratory (c/s figure-writing decrease alone in three or four limbs. 3. Moderate decrease in touch or pain or position sense, and/or essentially lost vibration in one or two limbs; or mild decrease in touch or pain and/or moderate decrease in all proprioceptive tests in three or four limbs. 4. Marked decrease in touch or pain or loss of proprioception, alone or combined, in one or two limbs; or moderate decrease in touch or pain and/or severe proprioceptive decrease in more than two limbs. 5. Loss (essentially) of sensation in one or two limbs; or moderate decrease in touch or pain and/or loss of proprioceptive for most of the body below the head. 6. Sensation essentially lost below the head. V. Unknown. |
| 5) Bladder-Bowel Functions (revised in 1982);
0. Normal. 1. Mild urinary hesitancy, urgency or retention. 2. Moderate hesitancy, urgency, retention of bladder or bowel, or rare urinary incontinence. 3. Frequent urinary incontinence. 4. In need of almost complete constant catheterization. 5. Loss of bladder and bowel function. V. Unknown. |
| 6) Visual (Optical) Functions;
0. Normal. 1. Scotoma with visual acuity (corrected) better than 20/30. 2. Worse eye with scotoma with maximal visual acuity (corrected) of 20/30 to 20/59. 3. Worse eye with large scotoma, or moderate decrease in fields, but with maximal visual acuity (corrected) of 20-60 to 20-99 4. Worse eye with a marked decrease in fields and maximal visual acuity (corrected) of 20/100-20/200; grade 3 plus maximal acuity of the better eye 5. Worse eye with maximal visual acuity (corrected) less than 20/200; grade 4 plus maximal acuity of the better eye of 20/60 or less. 6. Grade 5 plus maximal acuity of better eye of 20/60 or less. V. Unknown. X. Added to grades 0 to 6 for the presence of temporal pallor. |
| 7) Cerebral (Cognitive) Functions;
0. Normal. 1. Mood alteration only (does not affect D5S score). 2. Mild decrease in mentation. 3. Moderate decrease in mentation. 4. Marked decrease in mentation (chronic brain syndrome- moderate). 5. Dementia or chronic brain syndrome-severe or incompetent. V. Unknown. |
| 8) Other Functions;
0. None. 1. Any other neurologic findings attributed to MS (specify). V. Unknown |
Table 2: EDSS, reprinted from Piri Çınar B, Güven Yorgun Y(9)
| 1.0 | No disability, minimal signs in one FS |
| 1.5 | No disability, minimal signs in more than one FS |
| 2.0 | Minimal disability in one FS |
| 2.5 | Mild disability in one FS or minimal disability in two FS |
| 3.0 | Moderate disability in one FS, or mild disability in three or four FS. No impairment to walking |
| 3.5 | Moderate disability in one FS and more than minimal disability in several others. No impairment to walking |
| 4.0 | Significant disability but is self-sufficient and up and about some 12 hours a day. Able to walk without aid or rest for 500 m |
| 4.5 | Significant disability but up and about much of the day, able to work a full day, may otherwise have some limitation of full activity or require minimal assistance. Able to walk without aid or rest for 300 m |
| 5.0 | Disability severe enough to impair full daily activities and ability to work a full day without special provisions. Able to walk without aid or rest for 200 m |
| 5.5 | Disability severe enough to preclude full daily activities. Able to walk without aid or rest for 100 m |
| 6.0 | Requires a walking aid-cane, crutch, etc. -to walk about 100 m with or without resting |
| 6.5 | Requires two walking aids-pair of canes, crutches, etc. -to walk about 20 m without resting |
| 7.0 | Unable to walk beyond approximately 5 m even with aid. Essentially restricted to wheelchair; though wheels self in a standard wheelchair and transfers alone. Up and about in a wheelchair some 12 hours a day |
| 7.5 | Unable to take more than a few steps. Restricted to a wheelchair and may need aid in transferring. Can wheel self but cannot carry on in standard wheelchair for a full day and may require a motorised wheelchair |
| 8.0 | Essentially restricted to a bed or chair or pushed in a wheelchair. May be out of bed much of the day. Retains many self-care functions. Generally, has effective use of arms |
| 8.5 | Essentially restricted to bed much of the day. Has some effective use of arms and retains some self-care functions |
| 9.0 | Confined to bed. Can still communicate and eat |
| 9.5 | Confined to bed and dependent. Unable to communicate effectively or eat/swallow |
| 10.0 | Death due to MS |
Diagnosis
In general, the primary investigations used to diagnose multiple sclerosis include Magnetic Resonance Imaging (MRI), cerebrospinal fluid (CSF) analysis, visual evoked potential (VEP), and blood tests including hemogram, renal and liver function tests, lipid profile, vitamins and electrolytes levels, viral serology and autoantibodies present in other autoimmune diseases such as lupus.(10)Investigations and clinical findings are integrated within the 2017 McDonald criteria (Table 3), which should be used with caution, especially in patients with an atypical presentation, such as initial presentation of progressive form, in children and elderly, or other patients with high suspicion of other differential diagnoses. (11)
| Number of lesions with objective clinical evidence | Additional data needed for a diagnosis of multiple sclerosis | |
| ≥ 2 clinical attacks | ≥ 2 | None |
| ≥ 2 clinical attacks | 1 (as well as clear-cut historical evidence of a previous attack involving a lesion in a distinct anatomical location) | None |
| ≥ 2 clinical attacks | 1 | Dissemination in space demonstrated by an additional clinical attack implicating a different CNS site or by MRI |
| 1 clinical attack | ≥ 2 | Dissemination in time demonstrated by an additional clinical attack or byMRIS OR demonstration of CSF-specific oligocional bands |
| 1 clinical attack | 1 | Dissemination in space demonstrated by an additional clinical attack implicating a different NS site or by MRI AND Dissemination in time demonstrated by an additional clinical attack or by MRIS OR demonstration of CSF-specific oligoclonal bands |
Table 3: McDonald 2017 criteria, reprinted from Alan J Thompson et al. (11).
Aggressive MS
Aggressive MS can exhibit any of the clinical features and courses of MS(2)(3)(6), some clinical characteristics are related to aggressive MS including:
Frequent attacks and shorter inter-attack intervals
Shorter inter-attack intervals correlate with faster progression to disability, walking difficulty, and need for a cane. Unresponsiveness to treatment and frequent attacks, especially within the first year of therapy, correlate with poor prognosis. Each episode in the early few years increases the risk of reaching disability milestones, including limited walking distance, need for a cane, and single neurological system moderate disability.(3)
Incomplete recovery after attacks
Incomplete recovery after MS exacerbations were associated with reaching disability milestones more quickly. A ten-fold increase in reaching the milestones was shown in patients with incomplete recovery. The strongest predictor of walking disability after ten years was a higher EDSS score after exacerbations. (3)
Motor, sphincter, cognitive or multifocal nature of symptoms
An increased proportion of patients reaching disability milestones in patients with pyramidal, sphincter, cerebellar, or brain stem dysfunction. However, patients with sensory or visual symptoms had a better prognosis, but the effect of visual symptoms on prognosis was modest after controlling for male gender, age, and progressive disease onset. In addition, the relation between motor symptoms and age, gender, and progressive onset are confounding factors in the relationship between motor symptoms and disability progression. Cognitive symptoms also predicted a higher EDSS. Although not found in all studies, an onset of multifocal symptoms was associated with a higher risk of disability in many studies. (3)
Progressive disease onset
Progressive disease onset is strongly related to a more aggressive course of MS in which patients’ risk of disability is two times higher than those with a relapsing-remitting disease course. Patients reach disability milestones one-third to half of the time that patients with relapsing-remitting disease need. (3)
Increased disease severity
Patients with aggressive MS exhibit more severe relapses that can increase EDSS by one or more points, increase any functional system score by two or more points, or increase two or more functional system scores by one or more points. Patients with aggressive disease acquire disability more rapidly and are more prone to reach an EDSS of 3 or more within the first year. (2)
findings associated with aggressive multiple sclerosis, include the following:
MRI findings
A high number of T2-enhanced lesions (⩾20) 0r gadolinium-enhanced lesions (⩾2), and T1 black holes indicating tissue loss and lesions in the spinal cord can predict increased severity of multiple sclerosis. Furthermore, spinal cord lesions, atrophy, and decreased upper cervical surface area are highly related to higher EDSS scores. The development of more than one gadolinium-enhanced lesion or new T2 lesions at follow-up is associated with a worse disease course. Also, early atrophy in the brain, including the cortex, deep grey matter, thalamus, basal ganglia, or infratentorial regions, is associated with aggressive MS and faster progression to disability. In addition, smoldering lesions indicating delayed recovery, persistent inflammation, and neurodegeneration are used as markers for aggressive MS(2)(3)(5). Figure ( 1 )Figure( 2)

Figure 1: T2 MRI enhanced lesions, Case courtesy of Associate Professor Frank Gaillard,(12)
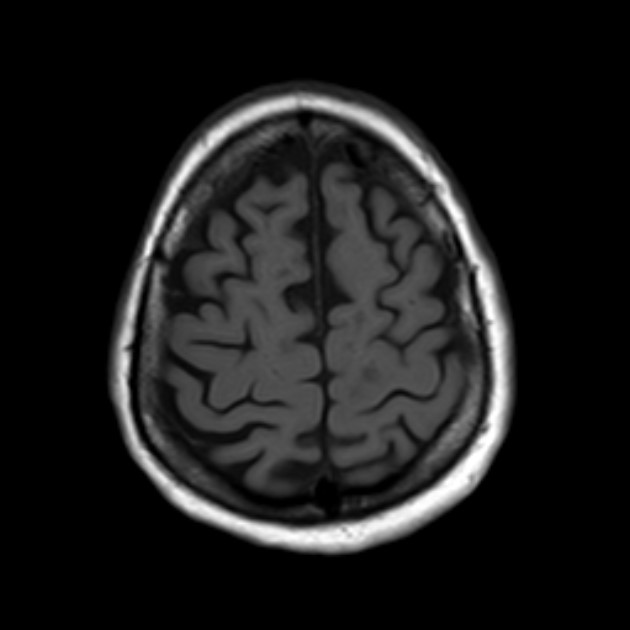
Figure 2: T1 black holes, Case courtesy of Dr Ahmed Abdrabou,(13)
Biochemical markers
Presence of IgM oligoclonal bands, B lymphocyte chemoattractant (CXCL13), neurofilament light chain (NFL), or Chitinase-3-like protein 1 (CHI3L1) in CSF, elevated IgG index and Elevated NFL in serum. (2)(3)(5)
There are many names and definitions used to describe aggressive multiple sclerosis for research or clinical purposes, and still no specific diagnostic criteria are agreed upon(2)(6), definitions include:
– Malignant MS: describes a very rapid progression of the disease, but the term is ambiguous because it is more commonly used to describe an unstoppable form of MS that can result in death within months to a few years, such as the Marburg variant of MS. Some researchers define malignant MS as rapid progression to death or disability. Another definition is the progression of EDSS to a score of 6 points in 5 years which is sometimes called ever malignant MS. Based on whether relapses or sustained progression is present, some research groups classify malignant MS into transient or sustained.(2)(6)
– Aggressive MS: has various definitions, including reaching an EDSS of 6 within five years, reaching an EDSS of 6 at 40, or transforming to secondary progressive MS within three years since relapsing-remitting MS onset. Other researchers use the definition of reaching an EDSS of 6 within ten years since disease onset. Aggressive MS is also defined as relapsing-remitting MS with one or more of an EDSS score of 4 within the first five years, two or more relapses in the last year with incomplete recovery, presence of more than two new or enlarging T2 or gadolinium-enhanced MRI lesions despite treatment or use of 1 or more disease-modifying therapies (DMTs) for one year with no response.(2)(6)
- Aggressive relapsing-remitting MS: where the baseline EDSS is 2.5-5, one or more gadolinium-enhancing lesions are present on MRI, and two or more relapses or an increase in EDSS of 2 or more points in the last 12 months. (2)(6)
– Aggressive onset MS is also used to describe patients having two or more relapses and two or more gadolinium-enhancing MRI lesions or having one relapse in the last year that results in a sustained increase of EDSS of 3 with two or more gadolinium-enhancing MRI lesions.(2)(6)
–Highly active MS is used to identify patients eligible for immunoablative therapy with homologous stem cell transplant and indicates a poor prognosis. It is defined as conventional treatment failure with one or more relapsed and incomplete recovery, MRI with one or more gadolinium-enhancing lesions of 3mm diameter or more, or an increase in T2 lesion of 0.3 or more per month in two consecutive MRI 6-12 months apart.(2)(6)
Multiple criteria are used to identify aggressive MS(3), as shown in Table 4, Table 5, and Table 6 below:
Table 4: Canadian working group assessment, reprinted from Dr James D. Bowen(3)
| Criteria | Low Level of concern | Moderate Level of concern | High Level of concern |
| Relapses | |||
| Rate | One in the second year of treatment | One in the first year of treatment | More than one in the first year of treatment |
| Severity | Mild:
Steroids not required Minimal effect on activities of daily living One functional domain affected No or mid motor/cerebella Involvement |
Moderate:
Steroids required Moderate effect on activities of daily living More than one functional domain affected Moderate motor/ cerebellar involvement |
Severe:
Steroids/hospitalization required Severe effect on activities of daily living More than one functional domain affected Severe motor/ cerebellar involvement |
| Recovery | Prompt recovery; no functional deficit | Incomplete recovery at 3 months; some functional impairment | Incomplete recovery at 6 months: functional impairment |
| Disability Progression | |||
| EDSS less than or equal 3.5
|
Less than or equal 1 point | 2 points at 6 months | >2 points at 6 months
2 points at 12 months |
| EDSS 4.0-5.0
|
<1 point | 1 point at 6 months | >1 point at 6 months
1 point at 12 months |
| EDSS more than or equal 5.5 | NA | 0.5 points at 6 months | >0.5 points at 6 months |
| Clinically documented progression | No motor symptoms; minor sensory symptoms | Some motor, cerebellar, or cognitive symptoms; multiple
EDSS domains affected |
Pronounced motor, cerebellar, or cognitive symptoms; multiple EDSS domains affected |
| Timed 25-foot walk | Less than or equal 20% confirmed at 6 months | >20% and <100% increase confirmed at 6 months | ≥ 100% increase confirmed at
6 months |
| MRI activity | |||
| New gadolinium-enhancing lesions
OR
Accumulation of new T2 lesions per year |
1 lesion | 2 lesions | ≥ 3 lesions |
Table 5: modified Rio score, reprinted from Dr James D. Bowen(3)
| Criteria
|
Points assigned |
| MRI done at 6 months | |
| ≤ 5 new T2 lesions | 0 |
| >5 new T2 lesions | 1 |
| MRI done at 1 year | |
| ≤ 4 new T2 lesions | 0 |
| >4 new T2 lesions | 1 |
| Relapse over 1 year | |
| 0 relapses | 0 |
| 1 relapse | 1 |
| ≥ 2 relapses | 2 |
Table 6: MS decision model, reprinted from Dr James D. Bowen (3). HADS: hospital anxiety and depression scale. If the color green was found on interpretation in all domains no change in therapy is needed, while patients with one yellow domain should be reassessed in three months and patients with two yellow domains or one red domain should have their therapies changed.
| Criteria | Points Assigned | Interpretation |
| Relapse | 0 points = green
1-4 points = yellow ≥ 5 points = red
|
|
| Each relapse | 3 | |
| Characteristics of relapses | ||
| Functionally relevant | 1 | |
| Residual symptoms after 3-6 months | 2 | |
| Interval since the start or change of therapy | ||
| More than or equal to 12 months | 0 | |
| 6-12 months | 1 | |
| 3-6 months | 2 | |
| Disability | 0 points = green
1 point = yellow ≥ 2 points = red |
|
| MS Functional Composite | ||
| Each test with worsening by 20% | 1 | |
| Each test worsened by 40% | 2 | |
| Symbol Digit Modality Test | ||
| Worsening by more than or equal 4 points | 1 | |
| Worsening by more than or equal 8 points | 2 | |
| Neuropsychology | 0 points = green
1 point =yellow ≥ 2 points = red |
|
| Fatigue Scale for Motor and Cognitive Functions | ||
| Worsening by 1 category | 1 | |
| Worsening by 2 categories | 2 | |
| Worsening by 3 categories | 3 | |
| Depression (HADS] | -1 | |
| Anxiety (HADS) | -1 | |
| Quality of life (MSIS-29) | No points | |
| MRI | 0-2 points = green
≥ 3 points = yellow |
|
| Each gadolinium-enhancing lesion | 1 | |
| Each new/enlarging T2 lesion | 1 |
An EDSS of 6 or more was associated with a secondary progressive disease which is of unfavorable response to aggressive treatment and hence can be used as a good criterion to identify aggressive MS.(6)
Differential diagnoses of MS that could also have aggressive presentation
Other inflammatory demyelinating diseases, some of which can be considered atypical forms of MS, such as Acute disseminated encephalomyelitis (ADEM) or acute hemorrhagic leukoencephalitis, Shidler disease, Balo concentric sclerosis, Marburg variant of MS, Tumefactive demyelination, Solitary sclerosis and Cavitary MS.(10)(14)
-Balo concentric sclerosis: Is a monophasic acute demyelinating disease most frequently affecting Asian women. Patients usually present with encephalopathy, headache, cognitive impairment, or seizures which are atypical of classical MS and might help differentiate the disease (10). Antibodies are usually negative, and patients are negative for oligoclonal bands. T2 enhanced MRI shows hyperintense alternating with a hypointense lamella,, which is related to regions of preserved myelin alternating with legions of severe demyelination. The disease is treated with corticosteroids and other immunosuppressants. The disease can be identified in patients with previous MS diagnoses, other atypical forms of MS,, or previously healthy patients. On pathology oligodendrocytes, loss and MS immune pattern III are seen (14). Figure (3)
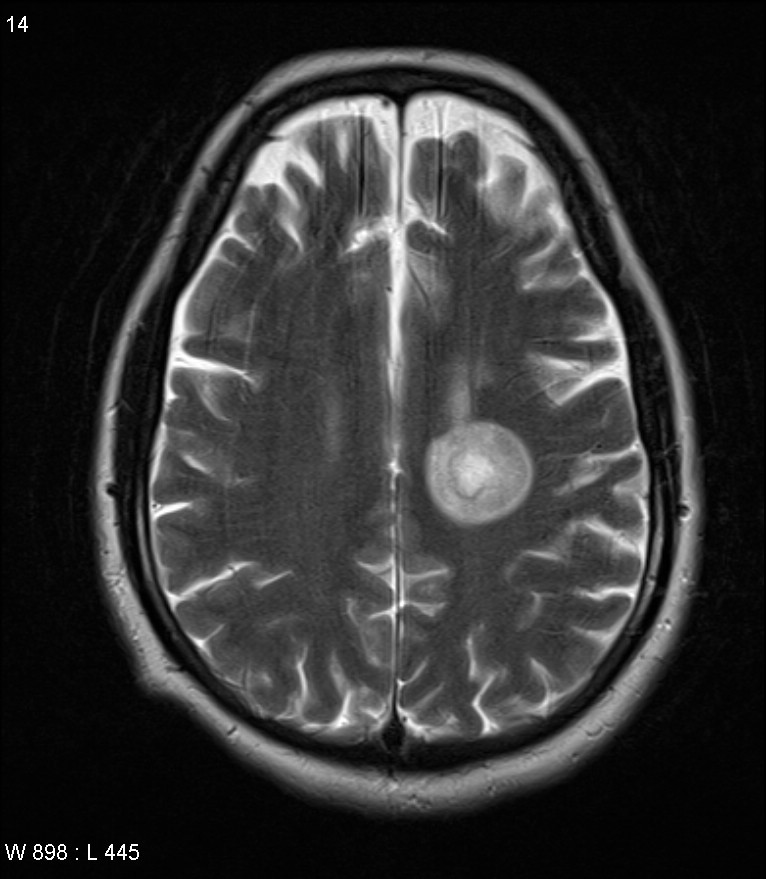
Figure 3: Balo concentric sclerosis on axial T2 enhanced MRI, Case courtesy of Austin Neuropathology(15)
– Tumefactive demyelination: patients usually present with atypical MS symptoms such as lethargy, headaches, and seizures. On MRI patients who have>2 cm well-defined demyelinating lesions (Figure 4) that can be isolated, multiple, or associated with other MS lesions. The lesions are ring-like or of infiltrative pattern and are in supratentorial areas. The oligoclonal band is an inconsistent finding in CSF analysis, but patients usually have higher CSF interleukin (IL)-6 and similar CSF IL-10 compared to MS. On histopathology, patients exhibit more Creutzfeldt Peter cells and dystrophic astrocytes, displayed in fewer numbers in typical MS (14). However, patients with tumefactive lesions have a favorable prognosis compared to other MS patients and are usually treated with corticosteroids or DMTS(16).
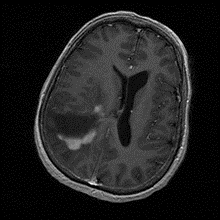
Figure 4: Tumefactive demyelinating lesion on MRI, Case courtesy of Dr Hani Makky Al Salam, (17)
-Schiller’s disease: Is another severe atypical MS variant that can be misdiagnosed as MS in the early stages because of the presence of white matter lesions. However, the disease progresses more rapidly and presents with impaired consciousness. On MRI, Schidler’s disease lesions are similar to tumefactive lesions. The disease affects children more frequently than adults .(10)
-Marburg variant of MS: This is a deadly atypical variant presenting with loss of consciousness, coma, and death within weeks. On MRI, supratentorial, infratentorial, and spinal cord lesions are present and progress rapidly. (Figure 5)
Marburg variant and Shidler’s disease have poor prognoses as corticosteroids or other immunosuppressants are ineffective in stopping disease progression .(14)
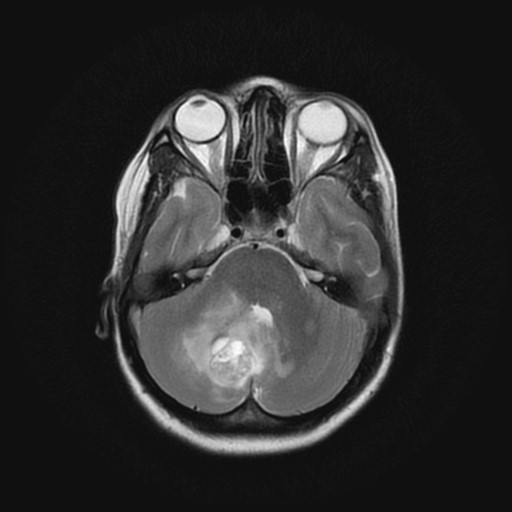
Figure 5: Marburg variant MS on MRI, Case courtesy of Dr Mohammad A. ElBeialy,{Formatting Citation}
Acute disseminated encephalomyelitis (ADEM): Is usually present in pediatric patients but rarely in adults. Patients typically present with acute or subacute encephalopathy and can progress to coma, in addition to fever, headache, fatigue, and nausea related to encephalitis. Furthermore, patients can have focal or multifocal symptoms according to sights affected by demyelination. The disease’s cause is idiopathic but related to a recent infection or vaccination. On MRI, multiple T2 hyperintense lesions are present bilaterally and asymmetrically (Figure 6). CSF analysis can show increased lymphocytes, an elevation in CSF pressure, mild protein elevation, normal glucose levels, elevated IgG index, and oligoclonal bands. The disease has a favorable prognosis and is usually monophasic, although some patients can have a non-monophasic presentation mainly related to MS. However, Acute hemorrhagic leukoencephalitis is a rare, severe variant with similar symptoms and a less favorable prognosis. The disease is distinguished by having hemorrhagic lesions . (10)(14)
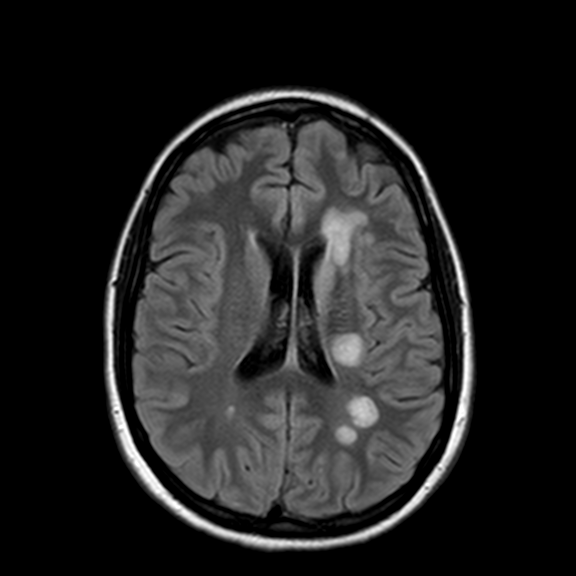
Figure 6: Axial FLAIR MRI Acute Disseminated Encephalomyelitis, Case courtesy of Assoc Prof Frank Gaillard,(19)
Solitary sclerosis: Is a progressive disease causing spastic paraparesis or hemiparesis. It is caused by an isolated demyelinating lesion in the corticomedullary junction which is found on MRI (Figure 7), through the involvement of other sites of the corticospinal tract. CSF analysis also shows oligoclonal bands. The disease is related to MS and can progress to MS. However, this is yet to be more thoroughly evaluated (14).
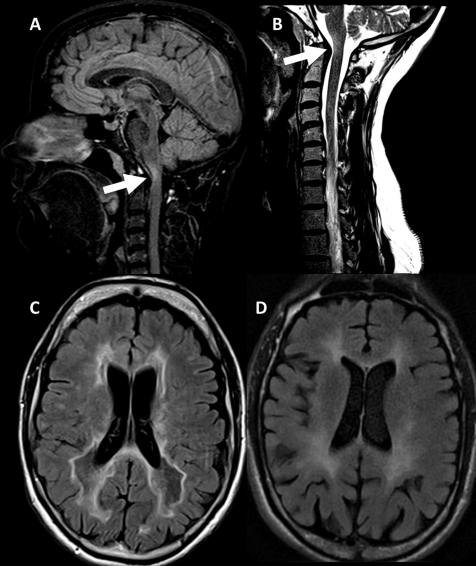
Figure 7: Solitary sclerosis on MRI, (A,B) showing lesions on cervicomedullary junction, C and D showing FLAIR hypointensities and hyperintensities respectively, reprinted from X. Ayrignac , C. Carra-Dallie`re and P. Labauge, (14).
Neuromyelitis Optica (Devic’s disease): This is a demyelinating disease that is no longer considered an atypical variant of MS(13). This disease is related to channelopathy due to antibodies against aquaporin-4 in the astrocyte foot processes. It presents with transverse myelitis and optic neuritis that occur simultaneously or separately. The disease is distinguished from MS by aquaporin-4 antibodies or anti-myelin oligodendrocyte glycoproteins. Also, the disease differs from MS by lack of remissions, rapid progression, and unresponsiveness to corticosteroids or other MS therapies; thus, the disease requires different treatments that can be more aggressive such as plasma exchange. (14)
Other non-demyelinating diseases to be considered as differential diagnoses to MS include inflammatory diseases such as systemic lupus erythematosus, Bechet disease, Sjogren syndrome, paraneoplastic syndromes, sarcoidosis and Wegener granulomatosis, infectious disease such Lyme disease, Human T-lymphotropic Virus-1 Associated Myelitis, CNS involvement in Human immunodeficiency virus, progressive multifocal leukoencephalopathy and neurosyphilis, in addition to metabolic disorders such as Adrenoleukodystrophies, Leber’s Hereditary Optic Neuropathy and Subacute Combined Degeneration. Physical examination and investigations are required to differentiate them from aggressive MS.(10)
Treatment
Patients with aggressive MS are unresponsive to conventional first- or second-line treatments of MS; thus, these patients mostly will have tried drugs that either block inflammatory cells’ access to the CNS, such as Natalizumab or that sequester these inflammatory cells in lymph nodes such as fingolimod. Second-line agents such as Natalizumab and fingolimod exert their effect if patients use these drugs. Natalizumab discontinuation is associated with severe, dangerous rebound disease, and prolonged use is associated with progressive multifocal leukoencephalopathy, although used anecdotally in some aggressive MS patients. On the other hand, fingolimod with a less severe rebound effect after discontinuation is not completely effective for aggressive MS. Therefore, therapies that eliminate immunoreactive cells, including alemtuzumab, rituximab, cladribine, mitoxantrone, cyclophosphamide or the use of immunoablative therapy and homologous stem cell transplant are more effective in aggressive MS . (3)(6)(21)
FDA-approved aggressive treatments for MS are shown in Table 7:
| Drug | Mechanism of action | Uses | Adverse effects | Monitoring |
| Alemtuzumab
|
Humanized monoclonal antibody against CD-52. It targets CD-52 on lymphocytes, leading to cell death and lyses. The different recovery rate of lymphocytes leads to a change in the lymphocyte profile, where TH1 and TH17 lymphocytes are depleted, and memory and regulatory T cells are increased(3)(6).
|
Approved for relapsing-remitting MS unresponsive to two or more treatments(3)(6).
|
Infusion reactions
Anaphylaxis
Infections include herpes virus, human papillomavirus, fungal infections, tuberculosis, and bacterial infections.
Autoimmune diseases include Graves’ disease, immune thrombocytopenia, autoimmune glomerulopathy, and Guillain-Barre syndrome.
Malignancies are the most common thyroid cancer and Melanoma.
Acute acalculous cholecystitis
Hypersensitivity pneumonitis and pneumonitis leading to lung fibrosis (3)(6)
|
A complete blood count (CBC), kidney function tests (KFT), Liver function tests (LFT), urinalysis, purified protein derivative (PPD) or QuantiFERON Gold test for tuberculosis and varicella-zoster titer should be done before drug administration. Also, baseline skin examination for skin cancers and vaccination is done before treatment.
During treatment, monthly CBC, KFT and urinalysis, quarterly thyroid function tests and thyroid examination for nodules, and annual human papillomavirus screening in women(6).
|
| Cladribine
|
A purine analog has antineoplastic and immunosuppressive effects and works selectively in lymphocytes, especially CD4+ and CD8+ lymphocytes leading to inhibition of DNA repair in these cells and cell death(3)(6).
|
Used in aggressive, relapsing-remitting MS and active secondary progressive MS unresponsive to other treatments(3)(6).
|
Increased risk of malignancies
Rash
Alopecia
Lymphopenia and neutropenia
Infections include varicella-zoster, hepatitis B, hepatitis C, tuberculosis reactivation, and progressive multifocal leukoencephalopathy.
Liver injury
Teratogenicity warrants the use of contraceptives until six months after the last dose.
There are case reports of myocarditis and acute heart failure (3)(6)
|
Screening for malignancies.
CBC and liver function tests before each dose and 2-6 months after each dose.
Varicella-zoster antibodies.
Hepatitis B and C, tuberculosis, and HIV screening.
Pregnancy tests and use of contraception(6)(22).
|
| Fingolimod
|
Binds all subtypes f sphingosine-1-phosphate receptor except type 2, causing these receptors to be internalized and degraded in the cells. This causes the inactivation of these receptors on naive lymphocytes, leading to the inability of these lymphocytes to exit lymph nodes and go into the circulation and to the CNS(3).
|
Approved for relapsing-remitting MS(3).
|
Cardiac side effects include bradycardia or atrioventricular block after the first dose. However, drugs that prolong QT interval should be stopped.
Herpes infections include herpes simplex encephalitis, varicella-zoster (shingles), and Kaposi sarcoma caused by the human herpesvirus 8. Other infections include cryptococcal meningitis, atypical mycobacteria, and progressive multifocal leukoencephalopathy.
Cutaneous malignancies include basal cell carcinoma, melanoma, and Merkel cell carcinoma.
Liver and respiratory side effects.
Macular edema.
Headaches
Back pain
Bloody diarrhea.
Elevated blood pressure
Posterior reversible encephalopathy syndrome(3).
|
An ECG should be done before and after the first dose, and hourly monitoring of pulse and blood pressure for 6 hours after the first dose.
Liver function tests every 3-6 months.
Ophthalmological evaluation every 3-4 months and when the patient reports visual problems.
Regular skin examination.
Varicella-zoster antibodies before treatment initiation. Baseline respiratory function tests.
Use of contraception during therapy(22).
|
| Siponimod
|
Binds sphingosine-1-phosphate receptors, subtypes 1 and 5(3).
|
Approved for the clinically isolated syndrome, relapsing-remitting, and active secondary progressive MS(3)
|
Like fingolimod(3)
|
Same monitoring as fingolimod in addition to genetic testing before treatment initiation.
Some variants of CYP450 metabolize Siponimod more slowly; thus, genotypic testing must be done before treatment. CYP2C9*1/*3 or *2/*3 genotypes should receive half of the dose, and CYP2C9*3/*3 homozygous patients should not receive the drug(3)(22).
|
| Natalizumab
|
A humanized monoclonal antibody against alpha-4 integrin subunit of adhesion molecules. Works by preventing endothelial adhesion of leukocytes, leading to impaired entry to target tissues, including the CNS(3).
|
Used for relapsing-remitting MS(3).
|
Hypersensitivity reactions and anaphylaxis.
Liver injury.
Malignancies include melanoma and primary central nervous system lymphoma.
Increased risk of infections, including primary multifocal leukoencephalopathy.
Rebound disease after stopping(3).
|
Brain MRI
CBC and LFT
JC virus antibodies (22)(23)
|
| Ocrelizumab
|
A humanized monoclonal antibody against CD-20. Works by fixing complement to target cells and causing cell lysis. Target B cells but not plasma cells, thus, don’t affect levels of antibodies but prevent B cells from presenting antigens to T cells (3).
|
Approved for primary progressive and relapsing-remitting MS(3).
|
Infusion reactions.
Hypogammaglobinemia
Infections include upper and lower respiratory infections, herpes viruses, hepatitis B reactivation, and progressive multifocal leukoencephalopathy.
Increased risk of malignancies including breast cancer(3).
|
Baseline CBC and LFT and every six months during treatment.
Vaccination.
Hepatitis B, hepatitis C, and tuberculosis screening.
Pregnancy tests and contraception.
Breast cancer screening
In patients with recurrent or severe infection, test for immunoglobulin levels(22).
|
| Mitoxantrone
|
An anthracenedione antineoplastic agent blocks type II topoisomerase, leading to disruption in DNA replication and repair. This causes inhibition of the proliferation of B and T cells and TH1-mediated release of cytokines(3)(6).
|
Higher benefit in young patients with more frequent relapses and lower EDSS, aggressive relapsing-remitting MS, and early secondary progressive MS(3)(6).
|
Gonadal dysfunction and amenorrhea in women.
Leukaemia and Cardiotoxicity, which lead to its use being less favorable recently.
Liver toxicity (3)(6)
|
Monitoring fertility and trying to preserve it.
Annual echocardiogram or mitigated acquisition scan for five years after treatment.
Doing blood counts every six months for five years.
Liver function tests
Monitoring of lifetime dose, which should not exceed 140mg/ m2 (6)
|
Table 7: FDA-approved aggressive MS drugs
Non-FDA-approved drugs for aggressive MS are shown in Table 8:
| Drug | Mechanism of action | Uses | Adverse effects | Monitoring |
| Cyclophosphamide | An alkylating agent used in cancer and autoimmune diseases has cytotoxic effects on a broad spectrum of B and T and works by cross-linking DNA leading to cell death independent of the cell cycle(3)(6).
|
Used in early secondary progressive MS, especially in young patients and aggressive MS(3)(6).
|
Hemorrhagic cystitis.
Gonadal toxicity.
Bladder cancer
Brain atrophy
Syndrome of the inappropriate release of ADH
Cardiotoxicity
Ototoxicity
Immunosuppression and myelosuppression
Nausea
Alopecia
Malignancies including hematological malignancies (3)(6)
|
Hemorrhagic cystitis can be prevented by adequate hydration, Mensa administration, and frequent voiding or bladder catheterization if needed
Every six months, do cytology and urinalysis, if cytology is abnormal, do annual cystoscopy.
Neurocognitive examination
Fertility monitoring and preservation(6).
|
| Rituximab
|
A partially humanized monoclonal antibody against CD-20 on B cells leads to B cell depletion with a mechanism similar to ocrelizumab(3)(6).
|
Used in relapsing-remitting MS. However, further studies are needed to confirm its efficacy in aggressive MS(3)(6).
|
Like side effects of ocrelizumab(3).
|
Like ocrelizumab(3).
|
Table 8: Non-FDA approved aggressive MS drugs
Immunoablative therapy with stem cell transplantation
It’s a non-FDA-approved approach used in autoimmune diseases, including MS, and is the most aggressive treatment of MS with high efficacy as there is no evidence of disease activity at 50 years approached 70%, which is twice as effective as other aggressive MS therapies. However, this increased efficacy is at the cost of increased risk of severe side effects, including allergic reactions and anaphylaxis, transplant reactions, myelosuppression, liver toxicity, and gastrointestinal toxicity, all of which depend on the type of therapy used. The more effective the therapy is, the more myelosuppression it will involve.(3)(6)(24)
This therapy involves multiple steps starting with stem cell mobilization using granulocyte colony-stimulating factor or immunosuppressants, leukapheresis to collect stem cells, and a conditioning regimen involving high-dose chemotherapy. Examples are:
Highly myeloablative busulfan plus anti-thymocyte globulin in which the risks of myelosuppression and veno-occlusive liver disease are high;
intermediate myeloablative therapy involving Carmustine, etoposide, cytarabine, and melphalan plus anti-thymocyte globulin (BEAM/ATG), which has an intermediate risk of myelosuppression;
nonmyeloablative therapy involves cyclophosphamide and anti-thymocyte globulin, whose side effects are mainly caused by cyclophosphamide. Finally, stem cell infusion leads to bone marrow reconstitution. This will lead to the elimination of autoreactive cells and a reset of the immune system. Immunoablative therapy with stem cell transplant was primarily effective in relapsing-remitting MS patients younger than 32 who didn’t use more than two other therapies. (3)(6)
Treatment approaches
The escalation approach is the first approach to treating aggressive MS (Figure 8). Treatment starts with less aggressive and effective drugs and escalates to higher efficacy drugs according to disease activity and responsiveness, thus, reducing the risk of unnecessary immunosuppression. The escalation approach is more effective in relapsing-remitting MS, but in aggressive MS, the narrow time window to progress to disability will make this approach less effective. However, replacing monotherapy of low-efficacy drugs with monotherapy of high-efficacy medications is found to be preferable to adding high-efficacy drugs to low-efficacy drugs as no increase in effectiveness was found, in addition to the fact that there is a risk of more side effects. The drug to be used next depends on the patient profile and which drugs were used before(6)(23).
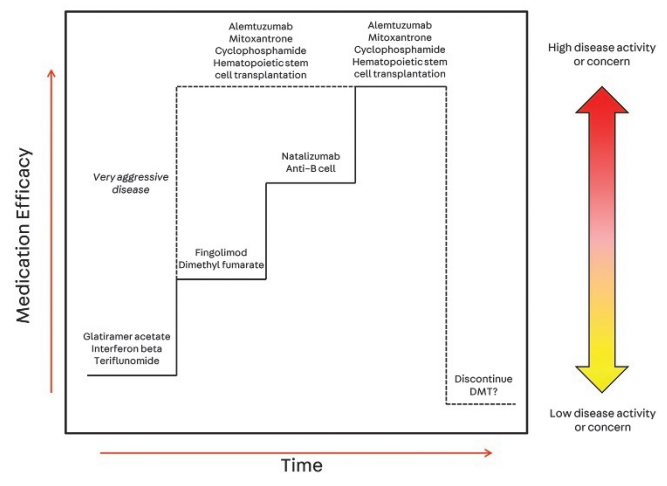
Figure 8: Escalation approach for aggressive MS, reprinted from Robert H. Gross, MD; John R. Corboy, MD, FAAN,(23)
The other approach is the induction approach, in which patients with high-risk features of aggressive MS start directly with high-efficacy treatments. This approach comes at the cost of increased toxicity of high-efficacy treatments. The induction approach can be done either by using an induction treatment for a specific period, such as potent lymphocyte-depleting agents including cyclophosphamide and mitoxantrone, alemtuzumab or hematopoietic stem cell transplant, then using a less aggressive maintenance DMT (Figure 9), or by using long term use of high efficacy treatments such as Natalizumab, or anti-CD-20 drugs without de-escalation(Figure 10). (6)(23)
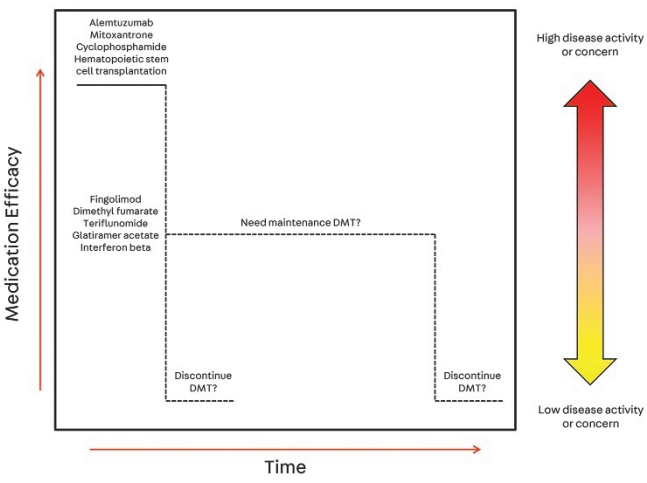
Figure 9: induction approach followed by maintenance, reprinted from Robert H. Gross, MD; John R. Corboy, MD, FAAN,(23)

Figure 10: maintenance doses of second-line agents, reprinted from Robert H. Gross, MD; John R. Corboy, MD, FAAN,(23)
Recommendations
Future retrospective studies or prospective studies that evaluate patients until fulfilling aggressive MS criteria should focus on arriving at a consensus on aggressive MS definition by confirming the best criteria to define patients early and use more aggressive treatments if needed. Risk factors for aggressive MS must be studied to predict patients requiring aggressive treatment. (2) More randomized controlled trials should be done to compare the effectiveness of different approaches in treating aggressive MS, drugs used within these approaches, and treatment algorithms. (6)(23) Also, an excellent endpoint to monitor patients’ response to aggressive MS treatment must be established. One proposed endpoint is NEDA (no evidence of disease activity), with no disease progression, new MRI lesions or new relapses, or a point close to NEDA. (6)











thanks for the article it helped me a lot to understand this disease