Article topic: High-Grade Glioma (Anaplastic Astrocytoma)
Author; Shroug Naser Al Hamdan
Editor: Ethar Hazaimeh
Keywords: anaplastic astrocytoma, malignant astrocytoma.
Overview
Anaplastic astrocytoma (AA) is a diffusely infiltrating, malignant, astrocytic, primary brain malignant tumor with a median age of onset of 41 years [1]. Presently, an anaplastic astrocytoma is defined by the histologic characteristics of nuclear atypia, increased cellularity, significant proliferative activity as manifested by mitoses, and lacking either endothelial proliferation or necrosis [1].
Nearly, a quarter of anaplastic astrocytoma (AA) arise as a de novo tumor, whereas it is estimated that three quarters are a consequence of transformation from a lower-grade astrocytoma[2].
AA accounts for 4% of all malignant CNS tumors and 10% of all gliomas[3]. The survival of patients with AA varies depending on molecular pathology. With conventional treatment, median overall survival and 5-year survival rates are 3 years and 28% [[3][4]].
Risk factors
Exposure to ionizing radiation and rare genetic syndromes such as neurofibromatosis type 1 and neurofibromatosis type 2, tuberous sclerosis, and the Li-Fraumeni syndrome is the only established risk factors.
Clinical presentation
Patients with AA mainly present with focal or generalized neurologic symptoms. The specific focal symptom depends upon the anatomic localization of this tumor and includes weakness, sensory loss, visual impairment, language dysfunction, and gait disorder. Generalized symptoms such as personality changes, seizures, and headaches. Compared to low-grade astrocytoma, seizure at presentation is less common [5].
Imaging
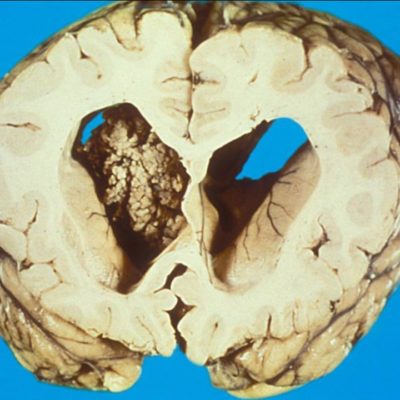
MRI with the administration of gadolinium contrast is the best imaging modality for diagnosis and management of Anaplastic astrocytoma. MRI helps in establishing a differential diagnosis, guides biopsy or resection is fundamental for treatment (i.e., radiotherapy) planning, monitoring response to treatment determines disease progression. MRI shows AA as an ill-defined, T1-weighted hypointense and T2-weighted hyperintense mass with surrounding vasogenic edema. Nodular areas of enhancement are usually observed, although nearly one-third of anaplastic astrocytoma display no contrast enhancement[2].
Contrast enhancement usually indicates the presence of a higher grade component notwithstanding if a biopsy indicates a low-grade tumor as sampling error may occur.
Advanced imaging includes diffusion-weighted MRI imaging, MR spectroscopy, MR perfusion, and amino acid positron emission tomography imaging may also help in the diagnosis and management of AA [[2]].
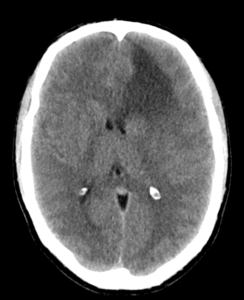
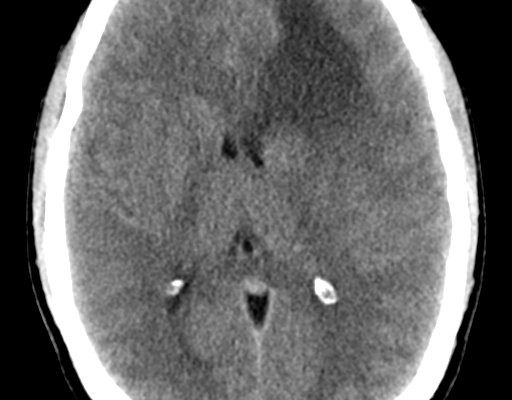
Figure (1): CT of anaplastic astrocytoma,Case courtesy of Dr. Bruno Di Muzio,[6]
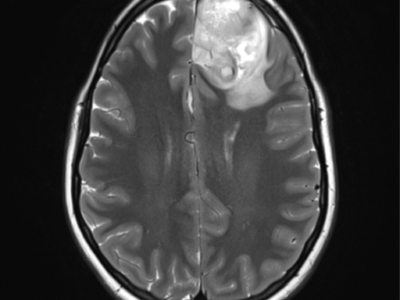
![Figure (2): MRI of ananplastic astrocytoma, Case courtesy of Dr Bruno Di Muzio,[7]](https://neuropedia.net/wp-content/uploads/2021/04/2-5-244x300.png)
Figure (2): MRI of anaplastic astrocytoma, Case courtesy of Dr. Bruno Di Muzio,[7]
AA frequently shows heterogeneous histology consisting of areas of low- and high-grade tumor that is thought to indicate progression from a lower grade tumor precursor. The diagnosis of AA is sometimes established from biopsy in which sampling error may occur. AA, defined by WHO as a grade III anaplastic glioma, characterized by mitotic activity, increased cellularity, presence of glial makers (e.g., GFAP), absence of neuronal markers, and nuclear atypia [1]. The MIB-1 labeling index in AA is 5–10% but may overlap with both low-grade astrocytoma and may show considerable variation within a given tumor [8]. The histologic classification and grading of anaplastic gliomas have poor benefit among pathologists and often poorly predicts clinical outcome [9]. NOW, clinicians are utilizing the molecular classification of gliomas to guide clinical decision-making.[10]
Types depending on molecular abnormalities
Anaplastic astrocytomas can be classified into more specific subtypes based on genetic characteristics. anaplastic astrocytomas can have abnormal genetic abnormalities, including mutations in the IDH1 or IDH2 genes. The presence of these genetic differences can affect prognosis and treatment, and are classified as:
- Anaplastic astrocytoma, IDH-mutant (if a mutation in either IDH1 or IDH2 )
- Anaplastic astrocytoma, IDH-wildtype (if no IDH mutations )
- Anaplastic astrocytoma, Not otherwise specified NOS (if the tumor has not been tested)[11]
Treatment
A personalized treatment plan will consider various factors, including size, location, and extent of surgical removal, among others.
- Surgery
Surgery is the first step in treating an anaplastic astrocytoma. The main objective is to remove as much of the tumor as possible while protecting critical brain function – this is called “maximal safe resection”, In some cases, doctors may be able to remove all the tumor or most of it.[5] However, anaplastic astrocytomas grow quickly, so doctors may only be able to safely remove part of the tumor, anaplastic astrocytomas can occur in near locations of the brain that control and manage body movement, sensation, language, or vision, special measures may be taken to protect these functions.[11]
Awake surgery with brain mapping is commonly used when tumors are located in the brain areas that control language or movement. This technique help surgeons safely identify and preserve critical brain regions.[5][11]
- Chemotherapy and radiation therapy
If the tumor can’t be removed with surgery, or only part of it was removed, it may need radiation therapy. Radiation therapy destroys rapidly dividing cells (cancerous cells ). This will assist to shrink the tumor or destroy any parts that weren’t removed during surgery.[11] Radiation may be suggested to treat residual tumor cells. Certain radiotherapy techniques such as Gamma Knife and IMRT offer the ability to specifically target tumor cells while reducing radiation exposure to healthy tissue, Chemotherapy may also be used to continue treatment after surgery, such as temozolomide (Temodar), during or after radiation therapy may be beneficial.[5][11]
EXTERNAL LINKS
https://www.neurosurgicalatlas.com/cases?query=anaplast