Article topic: Choroid Plexus Tumors
Author: Haneen Diab
Editor: Rahmeh Adel
Reviewer: Ethar Hazaimeh
Keywords: choroid plexus tumors, choroid plexus papilloma, choroid plexus carcinoma
Overview
The choroid plexus is a capillary network lined with specialized cells called ependyma. Ependymal cells, which are ciliated simple columnar glial cells that line the ventricles and central canal of the spinal cord, are necessary for CSF production. So, the choroid plexus can secrete up to 500 ml of CSF per day in the adult human brain.1 The choroid plexus, in collaboration with the arachnoid matter, forms a pair of membranes that separate the blood from the CSF. This barrier is made up of ependymal (choroid epithelial) cells with tight junctions on their apical surface (the side facing the ventricles), as well as a core of fenestrated capillaries surrounded by connective tissue. The blood-CSF barrier, like the blood-brain barrier (BBB), facilitates the exchange and removal of metabolites while preventing the passage of blood-borne substances into the brain. Microglia, white blood cells, macrophages, dendritic cells, and lymphocytes are all found in the choroid plexus and work together to keep harmful pathogens out.2
Choroid plexus tumors (CPTs) are rare and highly vascularized central nervous system neoplasms that account for 1% of all brain tumors,3 but 12-20% of brain tumors in the first year of life, male to female ratio is 1.2:1, The age at diagnosis range from 0 years (fetus) to 72 years, but the majority of the patients are children, resulting in median age at diagnosis of 3.5 years.4
According to the World Health Organization’s 2007 classification of Tumors of the Central Nervous System, choroid plexus tumors include choroid plexus papilloma (CPPs), atypical choroid plexus papilloma (aCPPs), and choroid plexus carcinomas (CPCs) 5
The majority of cases occur in the ventricles (the lateral ventricles being the most common). CPPs have a preference for the third and fourth ventricles compared to CPCs and are more likely to be found in older patients. Extra-ventricular cases have also been reported. 6 CPPs accounted for 74% of all CPTs according to a study from 2004 to 2009.7
Etiology and Pathophysiology
Some choroid plexus tumors have been linked to SV40 infections8, but they could also be influenced by other factors like X-chromosome-linked syndromes. Based on the findings of the study, it was hypothesized that SV40 is more common in infant tumors and that adult choroid plexus tumors have a distinct etiology.4 Aicardi syndrome, an X-linked genetic disorder characterized by agenesis of the corpus callosum, chorio-retinal lacunae, infantile spasms, and an increased risk of developing (sometimes multifocal) CPTs, is another rare genetic disease associated with CPTs.9
CPCs are strongly linked to Li-Fraumeni syndrome (LFS), a classic cancer predisposition disorder caused by TP53 tumor suppressor gene germline mutations.10
Histopathological feature
Choroid plexus tumors are classified as papilloma (grade I), atypical tumors (grade II), and carcinomas (grade III). by the World Health Organization (2016). CPPs have less than two mitotic figures per ten high power fields, atypical ones have two to five per ten high power fields, and carcinomas have more than five mitotic figures per ten high power fields.11
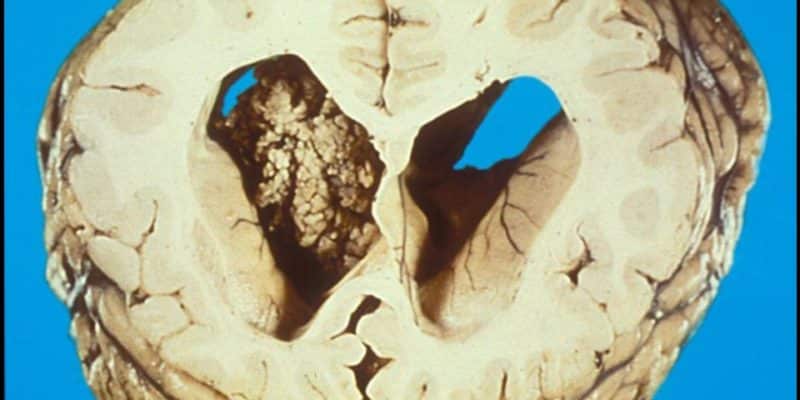
CPPs have a similar histological architecture to normal choroid plexus, but the cells are generally more crowded, elongated, or stratified. They have a fibrovascular stalk that is surrounded by a single layer of cuboidal to columnar epithelium that is arranged in a papillary pattern. 5 As shown in figure 1. CPPs are described macroscopically as pink-grey, pedunculated, cauliflower-like masses that are well circumscribed from the normal brain.12
CPCs, which are rarely confused with CPPs, are invasive lesions with overt signs of malignancy that include at least four of the following: increased cellularity, papillary architecture blurring, high mitotic activity (0.5 per 10 HPFs), nuclear pleomorphism, and necrosis.15
CPT shows an immunohistochemical expression of cytokeratin, vimentin, podoplanin, and S-100.13 Markers such as glial fibrillary acidic protein, transthyretin (prealbumin), and synaptophysin show variable expressivity; although CPPs are generally more common than CPCs. Kir7.1 and stanniocalcin-1 are both considered sensitive as well as specific CPP markers.14
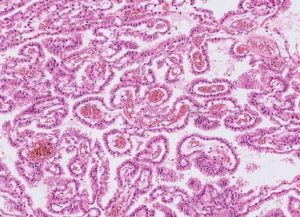
Figure1 : choroid plexus papilloma
CPPs are benign tumors with preserved papillary or finger-like architecture, with fibrovascular cores, lined by a single layer of cuboidal to the columnar epithelium, almost resembling nonneoplastic choroid plexus but with mild cellular atypia and without significant mitosis
Epigenetic dysregulation, including changes in DNA methylation, has long been recognized as a factor in tumorigenesis and tumor maintenance. Specific changes in DNA methylation in small genomic regions have been shown to be promising targets for the development of powerful prognostic and predictive biomarkers.16
CPTs have three distinct and molecular subgroups, according to DNA methylation profiling 17;
- Supratentorial pediatric choroid plexus tumors of low-risk (CPP and aCPP)
- Adults with infratentorial low-risk choroid plexus tumors (CPP and aCPP) and
- Supratentorial pediatric choroid plexus tumors of high risk (CPP and aCPP and CPC)
Epigenetic profiling will hopefully help us understand choroid plexus tumor biology, can be used to identify patients at risk of recurrence, and is expected to play an important role in treatment stratification and patient management in future clinical trials.18
Clinical presentations
The most common primary presenting symptom was a headache with nausea or vomiting caused by increased intracranial pressure, followed by ataxia and lower extremity weakness.19
Children’s symptoms include macrocephaly, splayed sutures, and tense fontanelles. The majority of these are caused by a combination of obstructive hydrocephalus, increased CSF production, and impaired CSF reabsorption at the arachnoid granulations due to scarring from hemorrhage or tumor debris.
Clinical characteristics and signs at the time of diagnosis were hydrocephalus, papilledema, gait impairment, and other conditions. Seizures, cerebellar signs, and psychomotor retardation are all symptoms of cranial nerve palsy.20 CSF rhinorrhea or hemifacial spasm may be the only clinical manifestations in some cases, particularly in fourth ventricle CPP.21, 22
Diagnosis and radiographic features
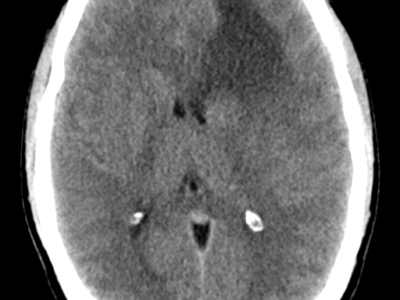
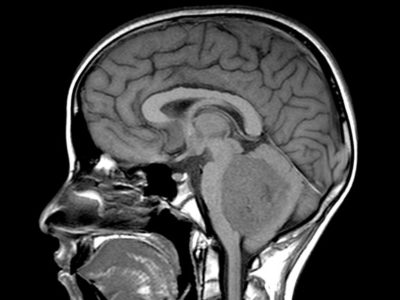
CPPs appear iso- or hyperdense on CT imaging, with calcifications in 25% of cases. Hydrocephalus is a common radiographic feature of these tumors and can be caused by increased CSF secretion or obstruction by tumor, hemorrhage, or debris. These lesions are well vascularized, brighten with contrast, and may have cystic features.23, 24 MRI revealed an enhancing mass in the ventricle that was isointense on T1-weighted images as shown in figure 2 but hyperintense on T2-weighted images.24
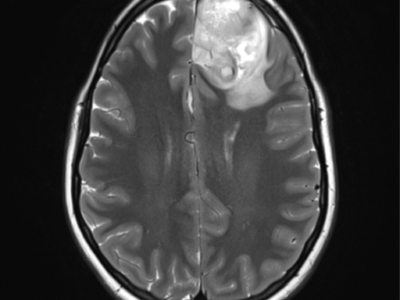
Recent research has shown that arterial spin labeling can help distinguish choroid plexus papilloma from choroid plexus carcinoma, ASL measured a higher rCBF in carcinomas, allowing differentiation between choroid plexus papilloma and carcinoma. As shown in figure 3.25
Prenatal ultrasound and fetal MRI were used in rare cases of prenatal CPP that revealed bilateral enlarged, echogenic choroid plexus with increased Doppler flow suggestive of vascularized choroid tissue.26
Angiograms are helpful to neurosurgeons in preoperative planning. Because these tumors are highly vascularized, intraoperative hemorrhage is a serious risk, particularly in pediatric patients who have a low threshold for intraoperative blood loss.27
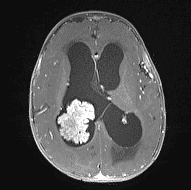
Figure 2: choroid plexus papilloma, MRI (T1 with Contrast + fat sat) 28
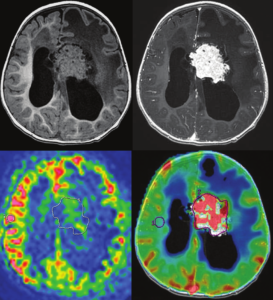
Figure 3: Measurement of ASL and DSC-PWI of a choroid plexus papilloma.
Top left: T1-weighted MR imaging. Top right: T1-weighted MR imaging with gadolinium injection. Bottom left: arterial spin-labeling. ROIs are drawn around the whole tumor and in the contralateral gray matter. Bottom right: DSC-PWI dynamic susceptibility contrast perfusion-weighted imaging. ROIs are drawn around the whole tumor and in the contralateral white matter. ROIs: regions of interest. 25
Treatment
Surgical resection
Surgery is still the most important and effective treatment for CPP patients. Survival has increased from 50% in early studies to nearly 100% in more recent reports, owing primarily to advances in imaging, surgical approaches, and intensive care quality.29
Several factors influence the overall outcome, including surgical complications, histological grading, age, lesion size, and location.30
The extent of resection is an important predictor of outcome, but the extent of resection depends on factors such as vascularity of the lesion, invasive nature of the lesion31, achieving gross total resection (GTR) is difficult, complications during surgery may occur due to the tumor’s hypervascularity, particularly intraoperative hemorrhage, which is a serious risk, particularly in pediatric patients with a low threshold for intraoperative blood loss 32
Preoperative embolization is an adjunct that is used in specific settings to reduce the vascularity of the lesions 31. Many studies have been published on the role of preoperative neoadjuvant therapy in reducing vascularity and aiding in gross total resection.33
Chemotherapy and radiotherapy
The CPT-SIOP-2000 study by the International Society of Pediatric Oncology reported on the use of etoposide, vincristine, and either carboplatin or cyclophosphamide for the treatment of various choroid plexus tumors.34
The role of radiosurgery in CPP management is unclear. it is not recommended for patients under the age of three, but it may be beneficial in treating recurrent or disseminated lesions or patients who are poor surgical candidates 35, 32
Prognosis
According to a study conducted at The Johns Hopkins Hospital, Patients with CPPs had 100% 5- and 10-year survival, while patients with CPCs had 71% 5-year survival.3
Long-term survival of patients with CPPs is expected because they are benign tumors though we suspected that tumor size might be related to patients’ prognosis 19
Complications
Postoperative vision changes, new onset seizures, unilateral weakness, and wound infection necessitating surgical debridement and wound revision were the most common complications in patients with CPPs.3
Recent clinical trials
A study provided one of the most detailed assessments of first and second cancer risks in Li-Fraumeni syndrome (LFS) patients published to date, the study found a high cumulative cancer risk, with females being more at risk than males, owing to early-onset breast cancer.
We also confirmed a recent finding that among children, brain cancer, osteosarcoma, Soft-tissue sarcomas (STS), and Adenoid cystic carcinoma (ACC) were the most common diagnoses, while breast cancer and STS were the most common diagnoses among adults.
Following the first cancer diagnosis, the risk of subsequent malignancies was approximately 50%, and it occurred from birth to 49 years later.36