Article Topic: Central Pontine Myelinolysis.
Author: Hosam Aldeen Katba Badar.
Editors: Almutazballlah Qablan, Sadeen Eid.
Reviewer: Ethar Hazaimeh
Keywords: pons, demyelination, hyponatremia, osmolyte, electrolyte.
Introduction
Central pontine myelinolysis (CPM) is a neurological disorder defined as well-formed symmetric damage to the myelin and to the oligodendrocytes in the pons with the preservation of neurons and axons. Extrapontine myelinolysis (EPM) on the other hand, is characterized by lesions in the thalamus, cerebral cortex, striatum, and other areas 1. Typical clinical manifestations of myelinolysis include paralysis, dysarthria, and altered mental status 2. Typically, CPM is secondary to the rapid correction of hyponatremia 1.
The condition was first reported by Adams and Victor in 1959, who reported “pontine myelinolysis” in alcoholic patients on pathophysiological and forensic findings 3. As an increasing number of manifestation sites in addition to the pons were detected, CPM and EPM were combined and are commonly referred to as osmotic demyelination syndrome (ODS)1.
Although early authors including Adams and Victors who first documented the condition focused on alcoholism as an etiology for CPM, it was not until the late 1970s that the association with electrolyte abnormalities 4 and the connection to rapid correction of hyponatremia was entrenched 5–7.
CPM is one manifestation of ODS. However, brain demyelination that is associated with the rapid correction of hyponatremia may be more diffuse and it is not always limited to the pons 8–12.
Etiology
There are many underlying causes and important comorbidities. All share the development of CPM as a consequence of a severe prior disease or its treatment. While most of the early publications described chronic alcohol use and alcohol withdrawal as being the critical comorbidity in more than 40% of cases, more recent work as well as some of the older literature places prior hyponatremia and the rapid correction of it as the most common cause, occurring in 30% to 78% of cases 13–16. Severe hyponatremia (serum sodium levels <120 mmol/L) is often present in around 47% of reported cases 17; however, the bulk of cases have additional concomitant cofactors, each of which alone can trigger CPM 14.
Hyponatremia is the leading pathology in the majority of cases, in addition to hyponatremia, other electrolyte imbalances can be the cause of CPM. General electrolyte disequilibria and osmotic imbalances have been reported, especially hypernatremia, hypophosphatemia, and hypokalemia 17.
Almost any electrolyte imbalance can be the causative agent. Severe hypokalemia is especially important as a sole determining factor as well as a cofactor, particularly for patients in intensive care units 17,18.
The brain’s adaptation to hyponatremia
The brain can adapt to hypotonicity and hyponatremia that develops over more than two to three days and is not associated with severe brain swelling; it is therefore much less likely to be complicated by seizures and comas, or brain herniations 8. However, once the brain has already adapted to hypotonicity, the rate of correction of hyponatremia becomes extremely significant. Rapid correction of hyponatremia and especially severe hyponatremia can lead to CPM.10 By contrast, rapid correction is not likely to induce CPM in patients with severe hyponatremia that has only been present for several hours or for a short amount of time since cerebral adaptation is at an early stage. An example includes acute water intoxication in marathon runners or psychotic patients, the rapid correction, in this case, does not usually lead to CPM in these patients. 8-12
The significance of the Duration of hyponatremia
Studies conducted on experimental animals showed that brain damage does not occur when hyponatremia of less than one day’s duration is swiftly corrected 19,20 nevertheless if hyponatremia persists for more than 2 days, the same exact treatment results in usually fatal demyelinating brain lesions. 19-29
Similar findings have been found in humans. Neurologic deterioration after a rapid correction of hyponatremia is improbable in patients with severe hyponatremia that develops over a few hours, especially in those whose normal water excretory capacity rapidly returns the serum sodium to a normal level. Examples include the ingestion of massive amounts of water over the span of several hours by marathon runners, psychotic patients, and users of ecstasy. 9,10,30,31
In these patients, hyponatremia develops swiftly, and the brain does not have time to fully adapt to the change in osmolarity. On the other hand, patients who develop hyponatremia over the span of several days have time for brain adaptation to occur and may develop CPM after rates of correction that are typically tolerated in patients with self-induced acute water intoxication. 8-12,30
Clinical presentation:
Clinical manifestations of CPM are highly variable. However, its time course is distinctive and central to diagnosis. Rapid elevation of the serum sodium concentration stereotypically precedes the process of demyelination and its symptoms by 1 to 14 days. The relatively stable clinical interval is then followed by CPM-induced secondary deterioration 14,32.
If the pons and the corticospinal and bulbar tracts are affected, patients present with recurrent encephalopathies and signs of insult to the brainstem14. With the involvement of the corticobulbar pathway, patients are affected by dysarthria and dysphagia (3.2% to 11.5% of cases), and brisk jaw jerk is commonly present. 14,11
If the corticospinal tracts are affected, initially flaccid and later spastic quadriparesis of varying severity materialize (in 9.8% to 28.8% of cases) this is usually seen in the first 5 days of the onset of symptoms, hyperreflexia, and bilateral Babinski signs are a common finding during this stage. 14,11
If tegmental lesions are present, ocular, and pupillary motility may also be affected (about 8% of all CPM). 14 In severe cases, the symptoms may lead to locked-in syndrome, a rare neurological disorder in which there is complete paralysis of all voluntary muscles except for the ones that control the movements of the eyes 32 Severe disorders of consciousness occur in 6.1% to 14% of cases. 14
Other common physical findings include increased muscle tone, facial weakness, and snout, grasp, or rooting reflexes. 11
Workup and diagnosis:
No correlation can be shaped between the diagnostic findings and the clinical outcome, mainly due to insufficient research evidence. 33,34
Laboratory workup
Knowing that the primary predisposing factors are electrolyte disturbances (especially the rapid correction of chronic hyponatremia). A suspected diagnosis of CPM should be supplemented by laboratory diagnostics and a good medical history. The laboratory parameters to be examined are serum sodium, serum potassium, and liver values (especially in the cases of previous liver transplants), as well as parameters for evaluation of the nutritional status (e.g., vitamin B12, methylmalonic acid, folic acid, and phosphate in serum). 35
Imaging
Magnetic resonance imaging
MRI is more sensitive than computed tomography for detecting the typical demyelination lesions in CPM. 36,37 Nonetheless, even an MRI may not become positive for as long as four weeks after the beginning of the disease. Therefore, an initially negative radiologic study in a patient who developed neurologic symptoms after overly rapid correction of hyponatremia does not exclude CPM, a repeat MRI should be obtained after several weeks. 37,38
There is no clear best timing of MRI imaging. A positive MRI finding can sometimes be present as recently as the first day after the onset of symptoms. 38,39 However, cases have also been reported where the MRI findings were initially unobtrusive and first pointed to the diagnosis at a follow-up examination several weeks later. 37,38
MRI is likewise beneficial in providing evidence for milder courses of the disease37,38. This has considerably improved the assessment and prognosis of CPM. 37
MRI course and sequences: Demyelination sites display hyperintensity in T2-weighted and T2-FLAIR sequences and show hypointensity in T1-weighted sequences, Occasionally gadolinium enhancement is also demonstrated (Figure 1) 36,40,41.

Figure (1A): Axial FLAIR, Oval lesion on pons that hyperintense on T2 and FLAIR, in CPM case. (62)
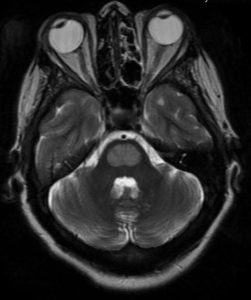
Figure (1B): Axial T2, Oval lesion on pons that has hyperintense on T2 and FLAIR. Symmetrical. (62)

Figure (1C): Coronal T1, Oval lesion on pons that has a hypointense signal on T1 (62)
Cranial computed tomography:
Cranial computed tomography (CCT) can also be used to confirm clinical CPM but has significantly lower sensitivity than MRI imaging. (initial detection rate of CCT: 25% to 28.5%). 36,39
Management
Patients who have developed CPM as a result of hyponatremia correction typically require intensive supportive therapy and should have their serum sodium re-lowered .51
Serum sodium re-lowering
The optimal target serum sodium and rate of relowering in patients with CPM have not been defined51. Data in humans are currently limited to case reports that suggest benefit from the early relowering of the serum sodium in patients who have developed symptoms of CPM. 42-45 As an example, a case report published in the journal of clinical nephrology described the case of a 71-year-old woman who developed symptomatic hyponatremia with serum sodium of 106 mEq/L in association with a thiazide diuretic. 43 The administration of isotonic saline solution was associated with an increase in serum sodium of 21 mEq/L in the first 24 hours. After initial neurologic improvement, the patient’s condition deteriorated after 72 hours. The administration of hypotonic fluid and desmopressin relowered serum sodium so that the final elevation was only 9 mEq/L. The patient then recovered without any neurologic sequelae .43
A similar result was documented in another case report in which serum sodium was raised by 23 mEq/L in the first 48 hours (figure 2). 44 After the onset of the neurological symptoms, the patient’s serum sodium was relowered from 132 to 120 mEq/L. the patient then had a full neurologic recovery over the ensuing 36 hours. 44

Figure 2: Reinduction of hyponatremia to treat central pontine myelinolysis 60.
Supportive therapy
Some patients with CPM recover functionally after extended periods of severe neurologic impairment .47 Therefore, aggressive supportive therapy should be provided to all patients who were functioning well prior to the onset of CPM47. Since patients with severe CPM commonly develop aspiration pneumonia and respiratory failure, endotracheal intubation and ventilator support are usually required. Recovery from apparently hopeless neurologic deficits does occur, thus, supportive therapy should be continued for a minimum of six to eight weeks before concluding that the newly acquired neurological deficits are irreversible 12,46,47.
Prevention
The severity of the initial electrolyte disturbance and the rate of its correction likely plays a critical role in developing CPM. In severe hyponatremia (<120 mmol/L), the incidence of CPM is substantially reduced by very slow correction of sodium, no more than <0.5 mmol/L per hour and <12 mmol/L per day.48 For hyponatremia (>120 mmol/L), the rate of sodium correction does not seem to play an important role in developing CPM. 32
The goal of treatment for hyponatremia is to initially achieve euvolemia, then to attain balanced osmolality. If hypokalemia is also present with hyponatremia, it must be treated first. 49
Depending on the cause of the hyponatremia, another potential approach is the use of vasopressin antagonists to enhance the elimination of water without concomitant loss of electrolytes. The selective V2 receptor antagonist ‘tolvaptan’ has been approved for this indication. 50 Due to the associated risks and a lack of outcome data 51, however, the use of this antagonist is reserved for severe refractory cases after careful consideration. 51 The risks include the danger of overcorrection, onset of hyperkalemia, and dangers associated with an initially critical, highly frequent monitoring of electrolyte and volume status. 51
Risk factors for developing CPM
Several risk factors appear to increase susceptibility to osmotic demyelination and CPM. When these factors are present, CPM can develop even at less severe degrees of hyponatremia and at slower rates of correction. 9,10,19,52-56 These include alcoholism, malnutrition, liver diseases, liver transplantation, and hypokalemia. 52,57 In a large study that included 83 patients with MRI-documented CPM, for example, 70% of the patients had an alcohol use disorder and 68% had hypokalemia. 52 Women of childbearing age are thought to be more vulnerable to fatal cerebral edema associated with acute hyponatremia. 58 Nevertheless, there is no evidence that gender increases the risk of CPM. 57
Prognosis
Up to the mid-1980s, CPM was thought to have a consistently poor outcome because the diagnosis was only confirmed by autopsy5. However, recent work has shown that good recovery from CPM is not rare and that total or near-total remission of the severe symptoms is possible. 47
Recent work describes a much brighter prognosis, with a good outcome in about 33% to 50% of cases. 34,36 About 24% to 39% of CPM patients reach normal neurological function; while another 16% to 34% are minorly affected and are self-sufficient in all activities of daily life. 59,34,37 In contrast, approximately 33% to 55% of patients will require some care, be fully dependent on care, or will die. 59,34,36
Predictors of a poor outcome were severe hyponatremia of <114 mmol/L and hyponatremia with hypokalemia14. Liver transplant patients who have clinically noticeable symptoms have the worst outcome. 14,61