Article Topic: Vagus nerve stimulation (VNS)
Author: Malek AlHourani
Edited: Almutazballlah Qablan, Sadeen Eid.
Reviewer: Ethar Hazaimeh
Keywords: vagus nerve stimulation, vagus nerve, refractory epilepsy, electrical stimulation
Overview of the vagus nerve
The Latin word vagus means ‘wandering’ and it somewhat explains the unique course of the 10th cranial nerve, the vagus nerve. The vagus nerve travels from the medulla to the colon mainly innervating the thoracic and abdominal organs. During this course, branches innervate various structures such as the larynx, pharynx, heart, lungs, and gastrointestinal tract. 1 The vagus nerve is a mixed nerve, composed predominantly of 80% afferent nerve fibers and the remaining 20 % efferent fibers. The afferent nerves transmit sensory information throughout the body to the brain, on the other hand, the efferent cholinergic fibers are the main parasympathetic component of the autonomic nervous system. 2
The afferent fibers terminate in the nucleus tractus solitarius, which is located in the brainstem. Then sends fibers that connect to different brain regions, such as but not limited to, the dorsal raphe nuclei, locus ceruleus, amygdala, hypothalamus, thalamus, and orbitofrontal cortex. 3
Vagus nerve stimulation brief history
Vagus nerve stimulation (VNS) can be used generally to describe any technique that will stimulate the vagus nerve.4
James L. Corning was an American neurologist who pioneered the first device for stimulating the vagus nerve to treat epilepsy towards the end of the 19th century. Despite Corning’s claims of dramatic benefit, his treatments were inconsistent and were ultimately not adopted by colleagues and were abandoned. VNS was not revived as a treatment for seizures until a century later. After safety and efficacy studies conducted in animals yielded positive results, evaluations of implanted devices for intermittent VNS in humans began in 1988. 5
In 1997, The US Food and Drug Administration (FDA) later approved an implanted left cervical VNS device for managing medically refractory partial-onset seizures in adults and adolescents. For some patients with partial-onset seizures, the side effects of antiepileptic drugs (AEDs) are intolerable; for other patients, no single AED or combination of anticonvulsant agents is effective. Although cerebral respective surgery is an alternative to pharmacotherapy in some cases, many patients with partial-onset seizures are not optimal candidates for intracranial surgery. So VNS presented itself as a promising new treatment for previously unmanageable cases. 6
Although VNS was not originally developed to treat depression, patients using VNS for epilepsy reported mood improvement; consequently, VNS was expanded to include the treatment of depression. Later in 2005, the same device was given an FDA-approved indication for the treatment of chronic treatment-resistant depression (chronic TRD). 7
Mechanism of action
At first glance, it is difficult to comprehend why a peripheral nerve stimulator like the VNS would be therapeutic for a central process like epilepsy or depression. Nonetheless, there is substantial evidence that the VNS is a device with significant benefits in these conditions.8 As of 2015, more than 100,000 VNS devices have been implanted. Despite the success of VNS to treat drug-resistant epilepsy, the mechanism underlying its effect is not completely understood. 8
The basic hypothesis on the mechanism of action was based on the knowledge that the vagus nerve afferent fibers have numerous projections within the central nervous system and that in this way generating action potentials in the afferent fibers will possibly affect the entire human being. 1
The ascending fibers will induce central effects on the brainstem structures (NTS, locus coeruleus, and raphe nuclei), and many of these potential mechanisms have been researched. 1
The locus coeruleus regulates central norepinephrine levels and VNS has been shown to increase the discharge rate of the locus coeruleus; subsequently increasing the central concentration of norepinephrine and increased levels of norepinephrine were associated with therapeutic efficacy. 9
A study has also shown that VNS increases the firing rate at the raphe nuclei; which are serotonin-secreting cells. 10 Increased serotonin levels have been correlated to decreased seizure activity. 11
Additionally, PET studies have shown increased blood perfusion to the thalamus and the primary somatosensory cortex and decreased blood perfusion to the limbic system in response to VNS therapy. 12 Furthermore, VNS raises the electrical current threshold needed to induce seizures. 12
VNS activation halts seizure activity in less than 5 seconds, 13 moreover, the anticonvulsant efficacy improves with long-term use when comparing results at 3 months to 1 year. 14 The combined acute and chronic effects of VNS most likely involve the recruitment of several different neuronal pathways and networks, both of which ultimately decrease seizure activity. 13,14
As for how VNS treats depression, it is mentioned previously that VNS alters levels of hormones famous for playing a role in regulating mood. Thus, it is easy to envision the VNS as a device to treat depression. 9
Current indications as per the FDA
Regarding epilepsy: The VNS Therapy System is indicated for use as an adjunctive therapy in reducing the frequency of seizures in patients 4 years of age and older with partial onset seizures that are refractory to antiepileptic medications. 15
Regarding depression: The VNS Therapy System is indicated for the adjunctive long-term treatment of chronic or recurrent depression for patients 18 years of age or older who are experiencing a major depressive episode and have not had an adequate response to four or more adequate antidepressant treatments. 16
Potential Uses
VNS therapy has wide-ranging effects. Due to the extensive effects on the nervous system, VNS therapy is being investigated as a possible treatment for a number of other disorders that impact the CNS, including migraine headaches, Alzheimer’s disease, and multiple sclerosis. 7
Several studies have shown that vagal nerve stimulation attenuates inflammation. 17,18 Inflammation is implicated in numerous conditions including cardiovascular disease, Rheumatoid arthritis, and Alzheimer’s disease. 19,20 Preliminary preclinical evidence suggests that VNS may reduce the inflammatory response by activating the cholinergic anti-inflammatory pathway (CAP). 7
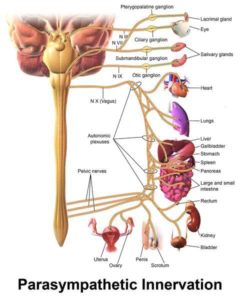
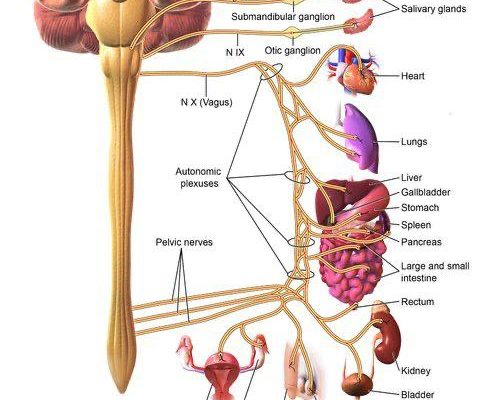
Fig 1 – Craniosacral distribution of the PNS; including the vagus nerve (33)
Right-sided VNS for the treatment of Heart failure showed therapeutic benefits in animal models. However, the two large, controlled trials-NECTAR-HF and INOVATE-HF-failed to demonstrate the expected benefit.21 The lack of successful translation of preclinical results to clinical application is most likely a result of inadequate activation of the nerve fibers that produce therapeutic effects. 21
The variety of changes brought about by VNS makes the potential applications of this device limitless, and it undoubtedly will be fascinating to watch what new findings come from future research.
VNS device implantation and complications
The most common use of VNS involves surgically implanting a commercially available programmable pulse generator device (NCP System; Cyberonics, Inc., Houston, TX, USA). The implant surgery is performed under general anesthesia in an outpatient setting. The electrodes which are made out of three spiral coils (anode, cathode, and anchor tether) are attached to the left vagus nerve where the superior and inferior cervical cardiac branches come off .22 Stimulation of these 2 cardiac branches may rarely cause bradycardia or asystole. 23
A systematic review analyzed the data from two large VNS studies from the late 1990s —namely the E03 trial and the E05 trial provide information regarding the complications experienced by patients receiving VNS therapy. The commonest side effect of high-stimulation VNS from the E03 trial was hoarseness of voice (reported by 37%), followed by throat pain (11%), coughing (7%), shortness of breath (6%), tingling (6%), and muscle pain (6%). From the E05 trial, shortness of breath, voice alteration, and pharyngitis were common side effects. Implantation site infection occurred in 2% to 7% of cases, where systemic antibiotics, device removal, or open wound debridement is required. Infection risk at the VNS implantation site in children is increased compared to adults. 24
Contraindications
The primary contraindication of VNS is baseline cardiac conduction disorders. Cardiac conduction abnormalities can worsen, especially on the right because there is the potential for efferent conduction through the vagus nerve. 25
Since the right vagus nerve supplies the sinoatrial node, the device is usually implanted in the left vagus nerve to prevent any cardiac dysrhythmias, therefore, VNS therapy is a contraindication in patients who underwent bilateral or left cervical vagotomy procedures. 26
Diathermy treatment specifically shortwave, microwave, and therapeutic ultrasound, could cause heating of the VNS system well above temperatures required for tissue destruction. As a result, it is contraindicated in people who have VNS implants. 4
VNS can reportedly induce or worsen sleep apnea syndrome so it can technically be considered a relative contraindication. 27
Non-invasive VNS
To avoid surgical implant-related complications, 2 types of non-invasive VNS were developed: transauricular and transcervical. The transauricular VNS (taVNS) stimulates the auricular branch of the vagus nerve that primarily supplies the concha and antihelix of the auricle. 28 The transcervical VNS (tcVNS) most likely stimulates both efferent and afferent VN fibers in the carotid sheath, moreover, tcVNS is approved by the FDA for the treatment of cluster headaches and migraines.29
Brain activity patterns after taVNS were somewhat similar to patterns observed in invasive VNS (eg, diminished activity in the thalamus, amygdala, and cingulate hippocampus). 30 NEMOS® is a new taVNS device that received the European clearance (CE mark) for epilepsy and is available in Germany, Austria, Switzerland, and Italy in 2010. 31
The gammaCore® device is a handheld tcVNS device under investigation for headaches, epilepsy, and gastrointestinal disorders. 31

Fig.2 – noninvasive VNS therapy 34
Transcutaneous VNS therapy warrants further study, not just as a treatment for depression or epilepsy, but also for its potentially favorable effects on the cardiovascular and nervous systems. 28
Conclusion
The safety and tolerability of implanted VNS therapy to treat epilepsy and depression patients have been well-established based on the research conducted by George L. Morris, III, and colleagues
VNS corresponds with a >50% seizure reduction in 55% of 470 children with generalized or partial epilepsy. VNS is associated with a >50% seizure reduction in 55% of 113 patients with Lennox-Gastaut syndrome. VNS is associated with an increase in ≥50% seizure frequency reduction rates of ∼7% from 1 to 5 years post-implantation. VNS is associated with a noteworthy improvement in mood scales in 31 adults with epilepsy. VNS is probably effective for both generalized or partial seizures and mood disorders in adults with epilepsy. VNS may have improved efficacy over time. 32
The extensive interface of the tenth cranial nerve, between the brain, body, gut, and heart, opens a plethora of therapeutic possibilities that will inevitably unlock many diseases in the following years.