Article topic: Acute Symptomatic Seizures
Author: Mohammed Belal Baker
Editors: Bashar Abualsiba’, Sadeen Eid
Reviewer: Ethar Hazaimeh
Keywords: Acute symptomatic seizure, provoked seizure, reactive seizure, situation-related seizure, epilepsy, unprovoked seizure
acute insult
Overview
Acute symptomatic seizures (also known as provoked seizure, reactive seizure, and situation-related seizure) is a common neurological disorder that occurs in close temporal relationship to a neurological or systemic insult1.
The International League Against Epilepsy (ILAE) has defined acute symptomatic seizures as: “events, occurring in close temporal relationship with an acute CNS insult, which may be metabolic, toxic, structural, infectious, or due to inflammation”1.
The interval between the insult and the seizure may vary according to the underlying clinical condition1 which will be discussed for each insult soon in this article. In addition, ILEA has recommended that the term acute symptomatic seizure should be used instead of provoked seizure, reactive seizure, or situation-related seizure1.
Epidemiology
According to population-based studies, the cumulative risk for acute symptomatic seizures from birth to 80 years of age was 3.6%2, whereas age-adjusted incidence was 29 to 39/100,000 person-years2,3. Acute symptomatic seizures comprise 40% of total seizures2 in addition to 34% of epileptic seizures2 And 40% of all first seizures3. It also represents 50%-70% of status epilepticus (SE) episodes4. Regarding gender-related distribution, males have twice the risk compared to females (5% until 80 years of age for males, in comparison to 2.7% for females)2. But this reflects sex-related differences in the incidence of underlying conditions, such as head trauma, rather than actual biological processes or phenomenon2.
The importance of the distinction between epilepsy and acute symptomatic seizures
As mentioned before, acute symptomatic seizures occur after acute CNS or systemic insult in a close temporal relationship, which helps to establish a direct causal association, and it has a low risk for recurrent seizures without a provocative insult in the future5, which is a characteristic feature for epilepsy.
However, the conceptual definition of epilepsy supported by ILEA define epilepsy as “a disorder characterized by an enduring predisposition to generate epileptic seizures and by neurobiological, cognitive, psychological and social consequences of this condition”. This definition requires the occurrence of at least one epileptic seizure6.
Whereas the operational or clinical definition of epilepsy by ILEA states that “epilepsy exists in a person who had at least two unprovoked seizures more than 24 hours apart or who had one unprovoked seizure and has a probability for the recurrence of further seizures that is similar to the recurrence risk after two unprovoked seizures (that is at least 60%) over the next 10 years”7.
Based on these descriptions, Acute symptomatic seizures differ from epilepsy in several important points. First, unlike epilepsy, the provocative factor of these seizures is clearly identifiable to the extent one can ever be certain of a causal association. Second, unlike epilepsy, acute symptomatic seizures are not necessarily characterized by a tendency for recurrence, excluding them from the new definition’s criteria of enduring predisposition to seize.
Furthermore, the importance to distinguish between the two categories arises from the fact that each has a different prognosis8 and management plan9, which necessitate the proper diagnosis and distinction between them.
Etiology
According to a population-based study done by Annegers et al.2 the most common and main causes of acute symptomatic seizures with their relative frequencies were as the following: acute stroke (16%), traumatic brain injury (TBI) (16%), CNS infections (15%), medications, alcohol and illicit drugs (14%), electrolyte and metabolic disorders (9%), encephalopathy (5%), and eclampsia (2%).
Pathogenesis
The underlying insults are responsible for decreasing the seizure threshold by altering the excitability of the neuronal membranes. an example of that is the way by which a stroke may induce a seizure. Acute symptomatic seizures after stroke are thought to be the result of acute biochemical dysfunction and excitatory neurotransmitter release leading to transient changes in neuronal excitability and electrically irritable tissue10. In addition, pro-excitatory cellular changes follow acute ischemic neuronal injury and include accumulation of intracellular calcium and sodium and increased extracellular concentrations of glutamate, which may lead to depolarization of the transmembrane potential and lower the seizure threshold10.
The mechanism of seizure initiation by hemorrhage is less clearly clarified. Blood products in the parenchyma and derivatives of heme and iron metabolism have been hypothesized to favor a focal cerebral irritation. Remarkably, the hemorrhagic transformation of an ischemic brain infarct is associated with a meaningful higher risk of acute symptomatic seizures than ischemic stroke alone, providing further support to the role of blood extravasation in the development of abnormal epileptiform activity11.
Regarding mechanisms responsible for medications-related seizures, it is almost related to decreased GABA effect. For example, penicillin (which was used to create animal models of epilepsy) prevents GABA from binding to the GABAA receptor12. Cephalosporins, imipenem, and fluoroquinolones antagonize GABAA receptors9. Isoniazid competes with pyridoxine, which is usually transformed into pyridoxal phosphate, a cofactor for GABA synthesis, thus leading to a decrease in GABA levels13. Regarding seizures resulting from withdrawal from barbiturates, benzodiazepines, and other sedatives, the downregulation of GABA receptors is probably the mechanism involved61.
Acute alcohol ingestion has an inhibitory effect on N-methyl-D-aspartate (NMDA) receptors, reducing excitatory glutamatergic transmission, and has an agonistic effect on GABAA receptors, Further, in chronic alcohol abuse, NMDA receptors are upregulated and GABAA receptors are downregulated, leading to tolerance. The roles are reversed during abstinence, with enhanced NMDA receptor function and reduced GABAergic transmission, leading to many of the symptoms and signs of acute withdrawal syndrome, including seizures15.
Age-related distribution
In general, despite age-specific distribution, the leading causes of acute symptomatic seizures were traumatic brain injury, cerebrovascular disease, drug withdrawal, and infection2. Regarding the age-specific distribution of acute symptomatic seizures by causes, it was shown that they are associated with the age distribution of CNS insults2.
Bacterial meningitis and intracranial hemorrhage were the major causes of acute symptomatic seizures in infants aged 1-6 months16, with Shaken-baby syndrome being the cause for all intracranial hemorrhage in infants aged 1-6 months16. Infections, metabolic causes, and encephalopathic seizures were the major causes during the first year of life2, whereas CNS infections and traumatic brain injuries were the major causes in the age group ranging from 1 year old to age 14 years2. Among young adult males aged 15-34 years, withdrawal and head trauma were the commonest causes2. The main cause for both sexes aged from age 35 to 64 years was drug withdrawal, followed by cerebrovascular disease, tumors, and trauma14. Cerebrovascular diseases were responsible for more than half of acute symptomatic seizures in elderly persons aged more than 65 years2.
Clinical criteria and time window accepted for each major cause
Seizures are considered acute symptomatic if they occur within the first 7 days of cerebrovascular disease1718; TBI (Traumatic Brain Injury), including intracranial surgery17,19 and CNS infections20. For CNS infections, acute symptomatic seizures can occur beyond 7 days, with persistent clinical or laboratory findings1.
For alcohol-induced seizures, the seizure must occur within 7–48 h of the last drink1. Seizures in the setting of acute alcohol intoxication (due to extremely high quantities consumed) are likely associated with that exposure14.
Alcohol withdrawal (AW) can be a provocative factor as well. Indicators of AW seizures include a history of chronic alcohol abuse, a history of current alcohol use with a recent reduction in consumption, and generalized tonic-clonic seizure with other symptoms of withdrawal such as tremors, sweats, or tachycardia (generally beginning within 5 to 10 h of decreasing ethanol intake); the seizure must occur within 7 to 48 hours of the last drink1.
For illicit drugs, seizures are considered acute symptomatic based upon the probability of seizure occurrence for specific drugs1. There is a high probability of cocaine and crack if metabolites are found in urine or blood; Normeperidine; Meperidine; Methaqualone; Glutarimide; stimulants (e.g., methylenedioxymethamphetamine) taken in excess; and inhalants1.
Furthermore, there is a fair probability for hallucinogens; angel dust (PCP, Phencyclidine) or (phencyclidine and Quatadine)1. However, there is low or no probability of heroin and marijuana1. The most common medications associated with seizures are antidepressants (bupropion, citalopram, venlafaxine, trimipramine, amitriptyline, maprotiline), antipsychotics (clozapine, chlorpromazine, quetiapine), antihistamines (diphenhydramine), and analgesics (tramadol and mefenamic acid)21–24.
Regarding metabolic disturbances, the propensity of metabolic disturbances to cause seizures may depend on the rapidity with which the disturbance develops: the more rapid the more likely it is to induce seizures25. Acute symptomatic seizures are operationally defined based on the blood sample obtained within 24 hours of the seizure when it is logical to assume that the findings are similar to those at the time of the seizure1. Cutoffs at the extreme end of abnormalities favoring specificity over sensitivity have been provided by the sub-commission on definitions for acute symptomatic seizure 1, which can be seen in table 1. The cutoffs are arbitrary and are only supported to some extent by the literature25,26.
When there is suspicion that the seizure may be an acute symptomatic seizure due to metabolic derangement, but the proposed cutoffs are not met, seizures should not be classified as acute symptomatic but instead be placed into an “unknown” category but excluded as epilepsy1.
| Biochemical parameter | Value |
| Serum glucose | <36 mg/dl (2.0 mM)or >450 mg/dl (25 mM) associated with ketoacidosis (whether or not there is long-standing diabetes) |
| Serum sodium | <115 mg/dl (<5 mM) |
| Serum calcium | <5.0 mg/dl (<1.2 mM) |
| Serum magnesium | <0.8 mg/dl (<0.3 mM) |
| Urea nitrogen | <100 mg/dl (>35.7 mM) |
| Creatine | >10.0 mg/dl (>884 lM) |
Table 1: Cut-off values for acute symptomatic seizures in metabolic disorders proposed by the International League against Epilepsy
Clinical presentation
Acute provoked seizures may result in obvious clinical seizure activity, subtle manifestations (i.e., gaze deviation, nystagmus, subtle twitching, altered mental status), or may not be associated with any clinical signs (i.e., nonconvulsive)11,27. Seizures are often seen in critically ill patients, particularly those in the neuro-ICU due to their underlying neurological injuries including TBI, intracranial hemorrhages, brain tumors, ischemic strokes, and CNS infections, etc.28. Numerous studies have revealed that approximately 20% of critical care patients have nonconvulsive electrographic seizures28,29. The majority of seizures (92%) in ICU patients are nonconvulsive28.
Complications
Specific studies investigating the detailed complications of acute symptomatic seizures are lacking. Although major negative outcomes depend largely on the underlying disorders9, some studies have found independent effects of acute symptomatic seizures on long-term outcomes and mortality. it was found that prolonged acute symptomatic seizures likely contribute to long-term outcomes by independently adding further brain injury to initial insults, where the main prognostic factors were etiology and severity of underlying brain injury30.
On the other hand, in a study of hospitalized patients, those who had a seizure for the first time in their life, most of them due to an acute symptomatic cause, had a significantly higher chance of a negative outcome (death or discharge to a hospice) than patients who already had a history of seizures prior to hospitalisation31. Cerebrovascular disease was found to be the most frequent etiology of seizures in these patients, followed by metabolic disturbances and brain tumors.
The other main complication is seizure recurrence after an acute symptomatic seizure, as the risk of subsequent seizures after an acute symptomatic seizure is substantial (33% for stroke, 13.4% for traumatic brain injury, and 16.6% for CNS infection) and almost approaches the recurrence risk for another unprovoked seizure after a first unprovoked seizure without underlying structural CNS disease32.
Work-up and diagnosis
Successful treatment of acute symptomatic seizures first requires that a seizure is recognized as acute symptomatic and that immediate diagnostic measures are undertaken to identify the underlying pathology. Information from the patient’s history and the findings of the physical and neurological examination will lead to the hypothesis that a seizure might have an acute symptomatic cause. This should then be further evaluated with a routine laboratory investigation and brain imaging, as well as further investigations such as EEG or a lumbar puncture if indicated. Once a diagnosis is made, proper treatment directed toward the underlying disease should be established as soon as possible.33
In stable patients, a detailed history of the condition should be taken. A witnessed account is particularly useful in describing the event if the patient is unresponsive, confused, or has amnesia. Associated neurological symptoms that should be inquired about include a history of headache, fever, photophobia, nausea, vomiting, neck stiffness, altered consciousness, head trauma, abnormal movements such as myoclonus or rhythmic shaking, the timing of onset, recent travel, sick contacts, history of alcohol or drug abuse, medications, and relevant past medical history27.
Further, a thorough general and neurological exam should be completed. The provider should look for signs of meningitis such as neck stiffness, photophobia, and Kernig’s and Brudzinski’s signs. Signs of increased intracranial pressure may be present, such as pupil dilation, sixth nerve palsy, or papilledema (particularly if subacute or chronic), observation of forced gaze deviation, myoclonus, facial twitching, altered consciousness, loss of bowel or bladder control, and nystagmus may be related to ongoing seizure activity27. Once seizure activity has resolved and the patient is stabilized, a laboratory work-up should include the following: complete blood cell count, basic metabolic panel, calcium, magnesium, creatine kinase, serum toxicology panel, urine toxicology panel, liver function tests, cardiac enzymes, urinalysis, blood cultures, and urine culture. Some of these tests (Table 227) are used to evaluate for underlying causes of the acute symptomatic seizures (i.e., urine toxicology screen), while others evaluate for potential seizure complications (i.e., cardiac and other muscle enzymes)27.
Fever may be present as a sign of underlying infection or as a symptom of convulsive seizures. If an underlying infectious etiology is suspected, a lumbar puncture should be performed. A lumbar puncture is also indicated if there is a concern for an autoimmune or paraneoplastic process27.
Consideration of diagnostic imaging should be done in conjunction with the initial laboratory workup. In an acute setting, a non-contrast computed tomography (CT) of the brain is a quick and easily accessible tool when evaluating for a stroke, ICH, SAH, SDH, epidural hematoma, TBI, neoplasm, or cerebral abscess. If the initial CT brain is unremarkable and the underlying etiology of the seizures is unknown, a magnetic resonance imaging (MRI) of the brain should be performed27.
EEG is indicated when an acute symptomatic seizure is prolonged, focal, or associated with persistent altered mental status. EEG obtained on an emergency basis may be necessary to assess for nonconvulsive status epilepticus in patients with cessation of convulsive movements but persistent altered mental status34.
A toxicology screen for frequently abused drugs is commonly obtained in patients with first-time seizures, but there is little data regarding its utility34.

Table 2: Diagnostic evaluation of acute seizures. Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
Treatment and management
Based on biological plausibility, the main approach to treat acute symptomatic seizures is to detect and then treat the underlying condition or insult that has provoked the seizure 9,33–35. If a patient has had a single seizure and is at a normal neurological baseline, likely no specific treatment is needed other than identifying and addressing any precipitating cause and assessing the risk for recurrence34.
There are specific circumstances to deal with. For example, if an underlying infectious etiology is suspected, immediate initiation of broad-spectrum IV antibiotics and IV acyclovir should be provided to empirically treat meningitis/ encephalitis, and a lumbar puncture should be performed.
The acute severe head injury also is a special circumstance. Even in patients who have not had a seizure, treatment for 1 week with an antiepileptic drug has been recommended with the intention of preventing early post-traumatic seizures34. Phenytoin was previously the drug of choice for this indication, but recent studies have suggested levetiracetam is equally effective and may have a preferred side effect profile34. If a seizure has occurred, longer antiepileptic therapy is generally employed34.
Treatment of seizures related to neuroleptics and antidepressants consists primarily of reducing dosage or changing to an alternative medication with a lower risk of seizures such as haloperidol, risperidone, selective serotonin reuptake inhibitors, or monoamine oxidase inhibitors36.
Managing unstable patients
Patients who have not returned to baseline should receive supplemental oxygen and be monitored for cardiac and pulmonary stability27. If the patient’s airway is compromised or if the patient becomes hypoxemic despite supplemental oxygen, then the patient should undergo intubation27. Rapid sequence intubation with a nondepolarizing neuromuscular blocking agent, such as cisatracurium or rocuronium, is preferred27.
Treatment with antiseizure medications (ASMs)
Patients with acute symptomatic seizures do not need to be treated with antiseizure medications (ASMs) on a long-term basis, although such treatment may be warranted on a short-term basis until the acute condition is resolved37.
Although patients with acute symptomatic seizures have a relatively lower risk for recurrence compared with unprovoked seizures8, the risk of mortality in the acute phase of the event is much higher8.for that reason, in cases where recurrence risk is high, patients with acute symptomatic seizures should be treated with ASMs during the acute phase of the underlying disease or as long as the precipitating factor or cause is present33,38. Patients with acute symptomatic seizures can develop further acute symptomatic seizures soon after the first event 39.
The aim of treatment with ASMs is to prevent further acute symptomatic seizures. However, data on how long it takes to treat patients with acute symptomatic seizures with ASMs is largely lacking. intravenous injection of fosphenytoin, phenytoin, levetiracetam, or phenobarbital is useful for patients who have difficulties in taking oral antiepileptic drugs35,40. Conventional oral antiepileptic drugs are useful for patients capable of oral intake.
If the seizure persists, we should treat for status epilepticus. Two algorithms have been proposed for the initial treatment of convulsive status epilepticus by the Neurocritical Care Society and the American Epilepsy Society27. The Neurocritical Care Society states that the treatment recommendations also apply to nonconvulsive status epilepticus27. Furthermore, both guidelines recommend rapid and emergent ASMs administration with a benzodiazepine27. The Neurocritical Care Society recommends that benzodiazepines be administered within 0 to 5 minutes, whereas the American Epilepsy Society recommends between 5 and 20 minutes27. Both recommend administration of IV lorazepam, intramuscular (IM) midazolam, or IV/rectal (PR) diazepam27. According to the Neurocritical Care Society guidelines, a repeat dose of the chosen benzodiazepine within 5 to 10 minutes can be administered if seizures continue37.
Chronic prophylactic use of ASMs should be avoided35,38, as the percentage of people who go on to develop epilepsy after acute symptomatic seizures are not great enough to justify it38. Their administration should be stopped after a short period because continuous administration does not prevent the transition to epilepsy35.
Prevention
Generally, prophylactic measures are directed toward the underlying condition. To elaborate, in case of suspected underlying infectious etiology, immediate initiation of broad-spectrum IV antibiotics and IV acyclovir should be provided to empirically treat meningitis/ encephalitis. To avoid withdrawal-provoked seizures, tapering of alcoholic and drug doses is needed.
It was found that phenytoin exerts a beneficial effect by reducing seizures only during the 1st week after severe head injury41. These findings certainly have largely contributed to the American Academy of Neurology’s recommendation for acute symptomatic seizure prophylaxis with phenytoin for 7 days after severe TBI42,43.
In another study, Carbamazepine has shown similar results to those of phenytoin—a reduction in early seizures but no effect on epilepsy even during the period of treatment44.
Risk factors
Recently, a large retrospective analysis has identified non-neurological infections, and a low premorbid functional level as shared risk factors for acute symptomatic seizures in hemorrhagic and ischemic stroke45,46, risk factors for seizures associated with CVT include supratentorial, particularly hemorrhagic, brain lesions, neurological motor and sensory deficits and thrombosis of the superior sagittal sinus and cortical veins47.
In the case of critically ill patients, the rate of seizures is higher for many subgroups such as postanoxic coma, brain tumors, severe TBI with intracranial hemorrhage, non-traumatic lobar intracerebral hemorrhage, central nervous system infections, and after convulsive SE48–50. Other risk factors for developing acute electrographic seizures include, for example, prior clinical seizures (recent or remote), coma, acute supratentorial brain injury, and sepsis33. Risk factors for acute seizures after TBI include more severe injury, need for neurosurgical intervention, depressed skull fracture, younger age (much higher in young children than adults), penetrating injury, and any type of intracranial hemorrhage33, as well as loss of consciousness or amnesia more than 30 minutes9.
Moreover, Encephalitis caused by herpes simplex virus (HSV) type 1 is the most common cause of sporadic encephalitis and the most strongly associated with acute symptomatic seizures in up to 60% in the acute stage33.
Table 3 summarizes the risk factors for acute symptomatic seizures with specific etiologies.
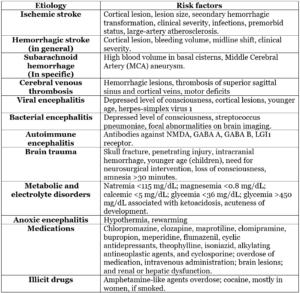
Table 3: Overview of the risk factors for acute symptomatic seizures with specific etiologies9,33.
Prognosis
Estimated risk of mortality after acute symptomatic seizures
The risk of early mortality differs between provoked and unprovoked seizures. it was found that patients who suffer from acute symptomatic seizures have a high risk for mortality in the weeks after the event51, furthermore, a study of two independent cohorts of patients with acute symptomatic seizures determined a case fatality rate of 20% in the first 30 days after the seizure51.
In a study of hospitalized patients, those who had a seizure for the first time in their life, most of them due to an acute symptomatic cause, had a significantly higher chance of a negative outcome (death or discharge to a hospice) than patients who already had a history of seizures prior to hospitalization52. Moreover, cerebrovascular disease was found to be the most frequent etiology of seizures in these patients, followed by metabolic disturbances and brain tumors. Another study compared the mortality of patients who had an acute symptomatic seizure with patients who had a first unprovoked seizure, moreover, those with acute symptomatic seizures had an 8.9-fold higher mortality rate in the first 30 days after the seizure event 51. However, no difference in mortality was evident after 10 years of follow-up between these two groups.
Whether acute symptomatic seizures themselves contribute negatively to a patient’s clinical outcome has been debated. This issue has been particularly studied in patients with stroke and some studies have demonstrated that acute symptomatic seizures are independently associated with higher mortality 53 while others have failed to show such an effect54.
However, in a large retrospective analysis of more than 1700 patients with acute symptomatic seizures, it was found that those with acute symptomatic seizures had almost twice the risk of in-hospital death compared to those without seizures, suggesting that at least for ischemic stroke, acute symptomatic seizures might indeed negatively influence a patient’s outcome independent of disease severity55.
Risk of seizure recurrence and epilepsy development after acute symptomatic seizures
The risk of subsequent unprovoked seizures after acute symptomatic seizures depends on the underlying insult16. Generally, a population-based study conducted in Southern Taiwan about acute symptomatic seizures in children showed that subsequent unprovoked seizures developed in 14% of the survivors of acute symptomatic seizures by age of 5 years16.
A recent study has shown that individuals whose first seizures were acute symptomatic seizures were 80% less likely to experience a subsequent unprovoked seizure but had higher early mortality, compared with individuals with a first remote symptomatic seizure when the etiology is stroke, traumatic brain injury (TBI), and CNS infection51. However, another study showed that the risk of subsequent seizures after an acute symptomatic seizure is substantial (33% for stroke, 13.4% for traumatic brain injury, and 16.6% for CNS infection) and almost approaches the recurrence risk for another unprovoked seizure after a first unprovoked seizure without underlying structural CNS disease56.
As mentioned before, the risk of further unprovoked seizures after provoked ones is determined by the underlying pathology. It was found that the risk of a first unprovoked seizure at 10 years of follow-up after an acute symptomatic seizure is 17% for structural causes, 17% for encephalopathic ones, and even higher after status epilepticus (SE) (41%), particularly if due to structural (45%) or encephalopathic causes (57%)57.
Recent updates
Association with COVID-19
A recently published hospital-based study conducted by Egyptian researchers58 showed an association between COVID-19 infection and acute seizures occurring in patients with multiple comorbidities, but previous thought that seizures are relatively rare neurological symptoms of COVID-19 infection59.
Discontinuation of ASMs in acute symptomatic neonatal seizure
A recent study included 303 neonates’ data with acute symptomatic seizures60 showed that no difference was found in functional neurodevelopment or epilepsy at age 24 months among children whose ASM was discontinued vs maintained at hospital discharge after resolution of acute symptomatic neonatal seizures, therefore, these results support discontinuation of ASM prior to hospital discharge for most infants with acute symptomatic neonatal seizures.