Title: Medial Medullary Syndrome
Authors: Denise Mourad, Elie Mourad
Editors: Omar Al Khatib, Christie Dib, Joseph Akiki
Reviewer: Hala Qaryouti
Keywords: Medial medullary syndrome, medulla, anterior spinal artery, hemiplegia, MRI
Abstract
Medial medullary syndrome (MMS) is a stroke syndrome affecting the medial medulla of the brain. It is caused by an atherothrombotic lesion of the paramedian branches of the anterior spinal artery, the vertebral artery, or the basilar artery. The syndrome is a triad of contralateral hemiplegia, contralateral sensory disturbance, and ipsilateral tongue deviation. Diagnosis is clinical and on diffusion-weighted and T2-weighted film MRI. Risk factors are the same as any other stroke syndrome and include hypertension, diabetes, dyslipidemia, and smoking. Management involves care in a stroke center using thrombolysis in the first 4.5 hours of symptom onset. Early rehabilitation is crucial to reduce residual damage of MMS. To avoid high morbidity and mortality, diagnosis and treatment should be prompt.
Overview
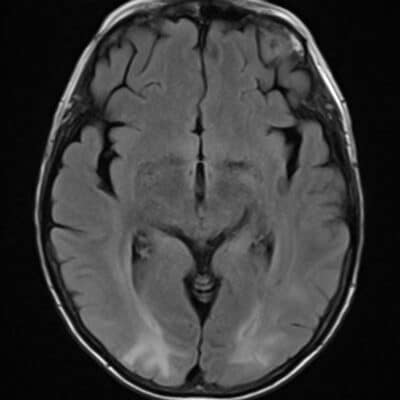
The medulla oblongata, or medulla (figure 1) for short, is a part of the brainstem made up of a ventral and a dorsal tegmentum. The medulla relays information from the spinal cord to the brain. The ventral tegmentum contains the pyramids and olives [1], while the dorsal tegmentum comprises the nuclei of the glossopharyngeal (IX), vagus (X), accessory (XI), and hypoglossal (XII) cranial nerves, as well as the continuation of the white matter tracts (i.e. medial longitudinal fasciculus, medial lemniscus, spinothalamic tract, central tegmental tract, spinocerebellar tract) [2].
At the pontomedullary junction, the medulla connects with the pons rostrally, and at the level of the C1 vertebrae, it connects with the spinal cord caudally. The pyramid, medial lemniscus, hypoglossal nucleus, and medial longitudinal fasciculus are medial medullary structures. These structures are supplied by the paramedian branches of the anterior spinal artery, a branch of the vertebral artery, which itself is a branch of the subclavian artery [3].
Stroke is the second leading cause of fatality in the world and a major source of disability. Occlusion of the vertebrobasilar artery (posterior circulation) causes 20 to 25% of ischemic strokes. A variety of neurological syndromes can occur in posterior circulation stroke, one of which is MMS, which represents less than 1% of ischemic strokes in the posterior circulation [4,5,6].
MMS, or Dejerine Syndrome, is caused by an ischemic stroke affecting the medial medulla of the brain. In 1908, William Spiller detailed the clinical and pathological characteristics of this syndrome, and in 1914, Joseph Dejerine named it “Interolivary or anterior bulbar syndrome” to characterize the famous triad of contralateral hemiplegia without facial involvement, deep sensation disturbance, and ipsilateral hypoglossal nerve palsy [7].
Figure 1 : Axial T2-weighted image (3 T) at the level of the medulla. V = cranial nerve V, IX = cranial nerve IX, X = cranial nerve X, XII = cranial nerve XII. [2]
Etiology and Pathogenesis
MMS is most frequently caused by infarction of the medial region of the medulla, brought on by an atherothrombotic occlusion of the paramedian branches of the anterior spinal, vertebral, or basilar arteries [8]. MMS can also result from dissection of the vertebral artery, especially in young patients [9].
As a result of the infarction, medial medullary structures like the hypoglossal nerve (the twelfth cranial nerve), medial lemniscus, and lateral corticospinal tract (figure 2) frequently suffer injury in MMS [3]. The spinothalamic tract is spared because it is located more laterally in the brainstem and is supplied by the vertebral and posterior inferior cerebellar arteries rather than the anterior spinal artery [10]. The trigeminal nucleus is also spared because the majority of it lies in the pons and its spinal part, which lies in the medulla, is lateral to the infarct [11].
The lateral corticospinal tract controls the voluntary movement of the body’s contralateral limbs. The contralateral nucleus cuneatus or nucleus gracilis sends sensory information including vibration, delicate touch, and proprioception, to the medial lemniscus which then relays it to the sensory cortex of the brain [12]. Hypoglossal nerve damage may occur if the caudal medulla is involved. Except for the palatoglossus, the hypoglossal nerve supplies the intrinsic and extrinsic muscles of the tongue. Therefore, the following muscles and their functions, respectively, would be impaired:
- Genioglossus: forward protrusion of the tongue from the root
- Hyoglossus: retraction and depression of the tongue
- Styloglossus: lifting lateral ends of the tongue.
- Internal muscles (transversus, verticalis, superior/inferior longitudinalis): alteration of the tongue form by curving, shortening, and narrowing it [13,14].
Figure 2: Top: Labeled anatomy of the medulla. IV ventricle= fourth ventricle. Bottom: Axial T2-weighted image (3 T) of the medulla. [2]
Risk Factors
Risk factors for MMS include [3,15,16]:
- Hypertension
- Dyslipidemia
- Diabetes
- Smoking
- Atrial fibrillation
- Atrial septal defect
- Patent foramen ovale
- Migraine
- Takayasu arteritis
Clinical Presentation and Complications
MMS is characterized by contralateral hemiplegia of the upper and lower limbs due to medullary pyramid involvement (corticospinal fibers of the pyramidal tract) [17,18]. Because the medial lemniscus is involved, there is a contralateral decrease in proprioception, vibration, and/or fine touch sensation [3]. Paresthesias or, less commonly, dysesthesias or “abnormal sensations” in the contralateral trunk and lower limb have been reported by some patients. The sensorimotor deficit seen excludes the face [8]. We can also find ipsilateral tongue deviation due to ipsilateral hypoglossal nerve damage [3] (the lower motor type of lesion). Even though tongue deviation is a feature of the MMS triad, it is less reported than sensorimotor dysfunction [3,19].
Although dysphagia is common in lateral medullary infarcts (LMI) patients, it is more common in bilateral medial medullary infarcts [20]. 11 cases of dysphagia or palatal palsy have been reported in a review of 28 cases of bilateral medial medullary infarct state [20]. One study showed that among 9 patients who had unilateral MMS, 78% suffered from dysphagia because of damage to the corticobulbar tract innervating the nucleus ambiguus [21]. It also showed that dysphagia is as frequent and severe in MMS as in LMI and that the characteristics of dysphagia are different between the two [21].
Other symptoms may include vertigo, dizziness [22], headache, vomiting [23], hyperalgesia, dysarthria, and disturbance in consciousness [6].
One study also showed that among 20 patients with MMS, some patients had ipsilesional upbeat nystagmus, others had ocular contrapulsion or contralesional ocular tilt reaction due to involvement of nucleus prepositus hypoglossi, mediallongitudinal fasciculus, and efferent fibers of vestibular nuclei and cells of paramedian tracts [24].
Bladder disturbances such as retention or incontinence were seen in bilateral MMS lesions [6].
Central post-stroke syndrome (CPSP) is defined as persistent pain with a visual numeric scale greater or equal to 4 in MMS [22]. In a study on 86 patients with MMS, around 25% suffered from CPSP described as a numb, cold, or painful sensation [22]. The symptoms were aggravated by climate changes and body movements. Patients with CPSP had poorer outcomes than those without CPSP [22].
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are the most common complications of MMS [3]. A PE can cause sudden onset dyspnea, which can result in death. Aspiration pneumonia can occur in patients with dysphagia seen in MMS [3]. If immediate treatment is not provided, the condition can be fatal. Patients suffering from hemiplegia may develop bedsores [25]. Patients can develop secondary infections over the bedsores which can lead to septicemia and death [20,26].
Workup and Diagnosis
Diagnosis of MMS involves obtaining relevant medical history, doing a thorough medical examination, and performing the relevant diagnostic tests.
In addition to searching for the relevant risk factors, complete neurological testing should be done because MMS is mostly a clinical diagnosis.
To rule out underlying atrial fibrillation, an ECG can be performed.
Serum glucose, serum electrolytes, a fasting lipid panel, and other baseline tests should be carried out as well [3].
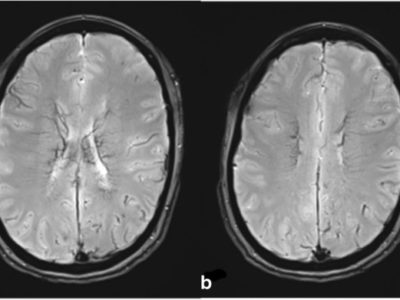
Imaging techniques include magnetic resonance imaging (MRI) or computed tomography (CT) scans to diagnose MMS. Compared to a CT scan, an MRI can more clearly show the lesion. The bony features on a CT scan may make it difficult to see the components of the posterior cerebral fossa. A hyperintense medial medulla lesion will be visible in diffusion-weighted (DWI) and T2-weighted film [8,27] (figures 3,4).
In a study showing the largest collection of MRI-identified MMS, the lesions mostly involved the rostral ventral area, which explains the high prevalence of motor dysfunction [22]. A magnetic resonance (MR) angiography or computed tomography (CT) angiogram can be used to pinpoint the location of the arterial blockage.
The following are the possible differential diagnoses:
- Multiple Sclerosis
- Hypoglossal nerve injury
- Posterior Cord Syndrome
- Intracranial tumor
- Advanced amyotrophic lateral sclerosis
- Hemimedullary infarction
- Brown-Sequard syndrome
Figure 3: Axial T2-weighted image (3 T) of the medulla shows the area involved in Dejerine syndrome (green). [2]
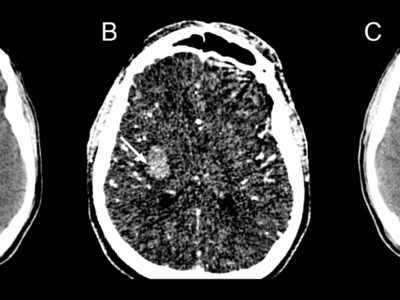
Figure 4: Findings of magnetic resonance diffusion-weighted imaging at the time of infarction onset. Infarction identified in the right-medial medulla oblongata on head magnetic resonance imaging (part marked by ∇). [35]
Treatment and Management
Management of MMS should be rapid just as with any other acute ischemic stroke. It should take place in a stroke center to reduce morbidity and mortality [3].
Before transporting the patient to the nearest stroke center, the following measures should be taken [3]:
- Assess ABCs and keep oxygen saturation >94%
- Put the patient under cardiac monitoring if possible
- Establish IV access and use normal saline to avoid cerebral edema
- Keep the patient NPO
- Treat hyperglycemia if present
If the patient reaches the hospital within 4.5 hours of symptom onset, initiate an intravenous (IV) recombinant tissue plasminogen activator (rtPA) [3,28]. After IV thrombolysis, patients should be transferred to the intensive care unit (ICU) or stroke unit for 24h [3,28]. Endovascular treatment is only indicated in patients with large vessel occlusion [3]. The options are intra-arterial fibrinolysis, mechanical clot aspiration, and angioplasty and stenting [3].
The infarcted area of the brain could cause impairment of cerebral autoregulation which could cause high blood pressure (BP) [3]. Permissive hypertension is allowed but BP should be treated if values are over 185/105 [28]. If this is the case, BP should be lowered by medications gradually. Treatment of comorbidities is important, especially hyperglycemia [3,29]. In the days following the thrombolysis, prevention of DVT should be established in immobilized patients using anticoagulants [30]. Although studies have shown that rehabilitation within 24 hours of the stroke event does not offer higher benefits [31], rehabilitation using physical and occupational therapy should be started early [3]. Swallowing assessment is crucial before the patient receives any oral medications or nutrition [3]. Since the placement of indwelling bladder catheters is associated with an increased risk of catheter-associated UTIs, it is recommended to avoid their use if possible [3]. A high clinical index of suspicion should be raised for aspiration pneumonia as these patients are at increased risk. Pulmonary complications could be reduced through frequent positional changes, postural drainage and suctioning, and chest physiotherapy [29]. After recovery, patients should be discharged on antiplatelets such as aspirin, and clopidogrel for secondary prevention [32]. They should also be put on statins [3].
Prevention
Primary prevention of stroke is done by controlling comorbidities like hypertension, dyslipidemia, and diabetes [26,33]. Important measures to control risk factors need to be done like weight loss, adopting a healthy lifestyle with a low-fat diet, and smoking cessation [26]. Secondary prevention of stroke is similar to primary prevention. The most crucial step to secondary prevention is to identify the cause of stroke to eliminate it [33]. In addition, patients should be discharged from the hospital on antiplatelets such as aspirin, clopidogrel, and statins [3]. If the origin of the stroke is atrial fibrillation, patients should be treated with oral anticoagulation [3].
Prognosis
The prognosis of the patient depends on the extension of the lesion and time to hospital presentation [3]. Stroke remains an important cause of disability and death worldwide but a substantial decrease in incidence, disability, and mortality has been observed in high-income countries [5]. Generally, MMS has a favorable prognosis if diagnosis, treatment, and initiation of rehabilitation are early [3]. If the patient has severe hemiparesis and hemisensory loss, residual motor, and sensory symptoms may last a lifetime [3]. Factors associated with a poor prognosis were age and severe hemiplegia in a study [22]. Severe cases were also seen among MMS with caudal (infarct in the central medulla) and ventral lesions [8]. A study of 26 previously reported cases with bilateral lesions associated with lingual paresis, quadriplegia, and respiratory symptoms also found a poor prognosis [34].
Recent Updates
In a study [35], a 66-year-old Japanese male patient who suffered from a medial medullary infarction, severe motor paralysis, intense numbness of the left arm, catastrophizing pain, and abnormal physical sensation was treated using a novel imagery neurofeedback-based multisensory systems (iNems) training method [35]. The researchers focused on the fact that, in addition to motor paralysis and numbness, this patient displayed a decline in physical representation in the brain. As a result, they created a training system (iNems) capable of sensing scalp-recorded nerve activity. They supplemented the existing rehabilitation program (upper and lower limb functional practice and practice of daily routine motion, walking, and dressing) with iNems training using EEG activity, which aimed to synchronize movement intent with sensory information [35]. The training was then performed for 6 weeks. As a result, significant improvements in motor function, catastrophizing pain, representation of the body in the brain, and abnormal physical sensations were achieved. iNems training also improved the neural activity of the default mode network at rest and the sensorimotor region when the movement was intended [35]. Therefore, given that both behavioral and neurophysiological changes were observed, the newly developed iNems could be a novel and useful tool for neurorehabilitation.
[5] M. Katan and A. Luft, “Global Burden of Stroke,” Semin Neurol, vol. 38, no. 2, 2018, doi: 10.1055/s-0038-1649503.
[6] C. Bassetti, J. Bogousslavsky, H. Mattle, and A. Bernasconi, “Medial medullary stroke: Report of seven patients and review of the literature,” Neurology, vol. 48, no. 4. 1997. doi: 10.1212/WNL.48.4.882.
[7] H. Sawada, N. Seriu, F. Udaka, and M. Kameyama, “Magnetic resonance imaging of medial medullary infarction,” Stroke, vol. 21, no. 6, 1990, doi: 10.1161/01.STR.21.6.963.
[8] T. Fukuoka et al., “Clinical review of 37 patients with medullary infarction,” Journal of Stroke and Cerebrovascular Diseases, vol. 21, no. 7, 2012, doi: 10.1016/j.jstrokecerebrovasdis.2011.01.008.
[9] D. Leppert and E. W. Radue, “Medial medullary syndrome due to vertebral artery dissection,” J Neurol Neurosurg Psychiatry, vol. 70, no. 1, 2001, doi: 10.1136/jnnp.70.1.130.
[10] C. J. Hodge and A. v Apkarian, “The spinothalamic tract.,” Crit Rev Neurobiol, vol. 5, no. 4, pp. 363–97, 1990.
[11] J. A. Brown, “The trigeminal complex. Anatomy and physiology.,” Neurosurg Clin N Am, vol. 8, no. 1, pp. 1–10, Jan. 1997, [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/9018701
[12] W. D. Willis and K. N. Westlund, “The role of the dorsal column pathway in visceral nociception,” Curr Pain Headache Rep, vol. 5, no. 1, 2001, doi: 10.1007/s11916-001-0006-1.
[13] H. C. Lin and P. E. Barkhaus, “Cranial nerve XII: The hypoglossal nerve,” Seminars in Neurology, vol. 29, no. 1. 2009. doi: 10.1055/s-0028-1124022.
[14] K. Javed, V. Reddy, and F. Lui, Neuroanatomy, Lateral Corticospinal Tract. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022.
[15] B. J. Sweeney and M. N. Rossor, “Medial medullary syndrome associated with patent foramen ovale in a weightlifter,” Eur Neurol, vol. 36, no. 6, 1996, doi: 10.1159/000117299.
[16] A. Deshpande, V. Chandran, A. Pai, S. Rao, and R. Shetty, “Bilateral medial medullary syndrome secondary to Takayasu arteritis,” BMJ Case Rep, 2013, doi: 10.1136/bcr-01-2012-5600.
[17] L. Caplan, “Posterior circulation ischemia: Then, now, and tomorrow: The Thomas Willis lecture – 2000,” Stroke, vol. 31, no. 8, 2000, doi: 10.1161/01.STR.31.8.2011.
[18] K. L. Tyler, E. Sandberg, and K. F. Baum, “Medial medullary syndrome and meningovascular syphilis: A case report in an HTV–infected man and a review of the literature,” Neurology, vol. 44, no. 12, 1994, doi: 10.1212/wnl.44.12.2231.
[19] K. Toyoda et al., “Medial medullary infarction: Analyses of eleven patients,” Neurology, vol. 47, no. 5, 1996, doi: 10.1212/WNL.47.5.1141.
[20] V. K. Paliwal, J. Kalita, and U. K. Misra, “Dysphagia in a patient with bilateral medial medullary infarcts,” Dysphagia, vol. 24, no. 3, 2009, doi: 10.1007/s00455-008-9194-8.
[21] M. Kwon, J. H. Lee, and J. S. Kim, “Dysphagia in unilateral medullary infarction: Lateral vs medial lesions,” Neurology, vol. 65, no. 5, 2005, doi: 10.1212/01.wnl.0000174441.39903.d8.
[22] J. S. Kim and Y. S. Han, “Medial medullary infarction: Clinical, imaging, and outcome study in 86 consecutive patients,” Stroke, vol. 40, no. 10, 2009, doi: 10.1161/STROKEAHA.109.559864.
[23] W. Kameda et al., “Lateral and Medial Medullary Infarction: A Comparative Analysis of 214Patients,” Stroke, vol. 35, no. 3, 2004, doi: 10.1161/01.STR.0000117570.41153.35.
[24] J. S. Kim et al., “Medial medullary infarction: Abnormal ocular motor findings,” Neurology, vol.65, no. 8, 2005, doi: 10.1212/01.wnl.0000180627.80595.10.
[25] A. B. Siddik and V. Gupta, StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022.Accessed: Jan. 10, 2023. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/2349601
[26] J. F. Meschia et al., “Guidelines for the primary prevention of stroke: A statement for healthcareprofessionals from the American heart association/American stroke association,” Stroke, vol. 45, no. 12, 2014, doi: 10.1161/STR.0000000000000046.
[27] K. Tokuoka et al., “A case of bilateral medial medullary infarction presenting with ‘heart appearance’ sign.,” Tokai J Exp Clin Med, vol. 32, no. 3, pp. 99–102, Sep. 2007.
[28] M. Alonso de Leciñana et al., “Guía para el tratamiento del infarto cerebral agudo,” Neurologia, vol. 29, no. 2. 2014. doi: 10.1016/j.nrl.2011.09.012.
[29] J. S. Balami, R. L. Chen, and A. M. Buchan, “Stroke syndromes and clinical management,” QJM, vol. 106, no. 7. 2013. doi: 10.1093/qjmed/hct057.
[30] E. C. Jauch et al., “Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association,” Stroke, vol. 44, no. 3, 2013, doi: 10.1161/STR.0b013e318284056a.
[31] L. A. Connell, N. B. Lincoln, and K. A. Radford, “Somatosensory impairment after stroke: Frequency of different deficits and their recovery,” Clin Rehabil, vol. 22, no. 8, 2008, doi: 10.1177/0269215508090674.
[32] M. Rahman and A. B. Siddik, Anatomy, Arterioles. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022.
[33] F. Z. Caprio and F. A. Sorond, “Cerebrovascular Disease: Primary and Secondary Stroke Prevention,” Medical Clinics of North America, vol. 103, no. 2. 2019. doi: 10.1016/j.mcna.2018.10.001.
[34] J. S. Kim, H. G. Kim, and C. S. Chung, “Medial medullary syndrome: Report of 18 new patients and a review of the literature,” Stroke, vol. 26, no. 9, 1995, doi: 10.1161/01.STR.26.9.1548.
[35] T. Kodama, O. Katayama, H. Nakano, T. Ueda, and S. Murata, “Treatment of Medial Medullary Infarction Using a Novel iNems Training: A Case Report and Literature Review,” Clin EEG Neurosci, vol. 50, no. 6, 2019, doi: 10.1177/1550059419840246.