Article topic: Cerebral Venous Thrombosis (CVT)
Author: Mira Hasan
Editors: Noor Qasem, Ethar Hazaimeh
Keywords: Stroke, emboli, anticoagulation, thrombophilia, antiphospholipid syndrome.
Introduction and epidemiology
Dural venous sinus thrombosis or thrombosis of cerebral veins is considered a special type of stroke [1], distinct from arterial stokes. Cerebral venous thrombosis (CVT) is less frequent than arterial stokes, and they target younger populations predominantly affecting females, rarely present as stroke syndrome, associated with a wider spectrum of clinical presentations which make the diagnosis even harder, and often delayed or missed, risk factors are also different from the ones known to be associated with arterial stokes, treatment options mainly parental heparin followed by oral anticoagulation, also associated with much less favorable factors [2] [3].
Low and middle-income countries have higher incidence due to higher pregnancy rates, increased infection rates, and nutritional deficiencies [4]. In some high-income countries, a study was conducted in the Netherlands showed an overall incidence of 1.32/100000/year and of 2.78/100000/year for women who are between 31 and 50 years [5]. Another study in Adelaide, Australia, using International Classification of Disease codes for CVT and neuroimaging reports, found the incidence to be of 1.57/100000/year, with a higher relative risk of 1.18 in females of reproductive ages [6]. A systematic review including 8829 CVT patients, from 74 case series including more than 40 subjects, found an average age of 32.9 years, along with a male to female ratio of (2:3).
This review showed a clear trend in the declining frequency of CVT patients who present with focal deficits or coma and a decrease in mortality over time. The decline in mortality can be owed to better management, and a decrease in septic CVT, but is mostly related to the recognition of less severe cases by MRI. Over time a shift in sex ratio was noted, with an increase in the proportion of women affected, possibly associated with an increase in the use of oral contraceptives [7]. Another study confirmed that in elderly patients, the sex ratio is evenly distributed, and is associated with a poorer prognosis than in younger CVT patients [8].
Risk factors and triggers
A condition associated with CVT can be classified into two groups, the first is predisposing like antiphospholipid syndrome, genetic prothrombotic diseases, the second is precipitant like oral contraceptive pills (OCPs) use, infections. They cannot be considered as causes of CVT because they do not fulfill the causality principles [4].
A multicenter case-control study compared women under 50 years of age, who suffered a CVT with historical controls to clarify the association between CVT and pregnancy along with puerperium, to revile no association with pregnancy but increased risk during postpartum especially during the first 6 weeks [9]. Using the same method, another study reported an increased risk of CVT in obese women reference. For women who are obese or overweight, the use of OCPs showed an increased risk of CVT in a dose-dependent manner [4]. Cancer is also a strong risk factor for CVT specifically within the first year of diagnosis and hematological malignancies [10]. In leukemic patients treated with asparaginase, high dose steroid regimens, intrathecal methotrexate injections in acute lymphocytic leukemia (ALL), are considered risk factors for CVT in these patients [11] two references, also all-trans-retinoic acid use [12]. The world health organization also confirmed anemia as a risk factor [13]. They found that anemia itself is associated with unfavorable factors for CVT [4]. Patients found to have higher levels of VIII a few months following the acute period were more frequent in CVT patients, which indicates it might be a risk factor too [14]. VENOST study in turkey found that Behçet’s disease was responsible for CVT in 9.4% of the patients. Most of the patients had a subacute course along with an intracranial hypertension syndrome, associated with thrombosis of the transverse sinus [15].
Clinical features
Signs and symptoms of CVT can be grouped into presenting syndromes, the most frequent ones are isolated intracranial hypertension syndrome, focal syndrome, and encephalopathy [16]. Less frequent ones are cavernous sinus thrombosis syndromes, syndromes of multiple palsies of the lower CN. In the conducted VENOST study, a large Turkish multicenter retrospective study, they collected 1144 patients to find the onset was acute in 47%, subacute in 34%, and chronic in 19%. Most frequently seen clinical symptoms and signs were headache (87%), which was isolated in 25%, nausea, and vomiting in 28%, seizures in 24%, visual field defects in 27%, other focal neurological deficits in 18%, altered consciousness in 18% and cranial nerve palsies in 18% [17].
So, the clinical picture varies accordingly to patients’ age. In elderlies, encephalopathy was more frequent, with fewer reporting headaches [8][16]. Another factor affecting the presentation of CVT is cerebral venous topography of the thrombosis. A systemic review of 116 patients with cortical venous thrombosis [18] complained mostly of headache, seizures, and focal neurological deficits.
Few patients presented with non-aneurysmal subarachnoid hemorrhage, either generalized or localized to a single or few cortical sulci of the hemisphere [19][20], or to perimesencephalic cisterns [21]. In a retrospective review of 138 patients with CVT [22], a subarachnoid hemorrhage in the absence of venous infarct happened only in 3 patients (2.2%). While in CVT patients with generalized subarachnoid hemorrhage presented with headache and neck stiffness, those with convexity subarachnoid hemorrhage have more complex symptomatology. In a series of 10 cases of convexity subarachnoid hemorrhage associated with CVT, all were related to superior sagittal sinus thrombosis. All patients complained of headache, 9 experienced seizures, 4 had papilledema, and only 4 complained of meningeal signs [19].
Seizures can also be focal or generalized from the onset and may progress to status epilepticus. In a large administrative US database, 16% of hospitalized CVT patients experienced seizures [23]. Acute symptomatic seizures are more frequently seen in CVT patients with supratentorial lesions, particularly if hemorrhagic, in those with motor and sensory deficits, and in cases with thrombosis of the superior sagittal sinus or cortical veins [24][25][26][27]. CVT patients can also present with status epilepticus, or status epilepticus may develop, particularly in patients with severe forms of CVT with supratentorial lesions, especially if multiple and hemorrhagic. Status may be refractory in about 1/6 of the patients, with refractoriness being related to the pretreatment duration of status [28]. At 6 months, 84% of CVT patients who had status epilepticus had a good recovery. Status epilepticus was not an independent predictor of poor outcome [4].
Few patients have experienced severe presentation as encephalopathy or coma and may need intensive care [29][30]. These patients also have decreased consciousness, altered mental status, bilateral or multifocal signs, and/or seizures or status epilepticus. They usually have multiple sinus occlusion, specifically the superior sagittal sinus and the deep cerebral vein system, also bilateral parenchymal lesions were seen, diffuse brain edema, or large herniating lesions. In a German multicenter neurocritical unit study of 114 CVT comatose patients, 1/3 of them recovered fully (mRS 0–1), 10% were severely disabled, and 1/3 died [4]. Clinical deterioration after admission, midline shift, and age predicted poorer outcomes [29].
Clinical scores may be useful to predict the outcome after CVT [31]. Barboza et al. [32] derived a CVT grading scale from a large multicenter Mexican series. The score has is composed of the following points, parenchymal lesion size >6 cm (3 points), bilateral Babinski signs (3 points), male sex (2 points), parenchymal hemorrhage (2 points), and level of consciousness (3 points). The above-mentioned score does not include the associated conditions in the prediction of outcome and needs validation in an independent sample.
Diagnosis
CVT diagnosis is can be difficult to make [33], once suspected an emergent neuroimaging is required [34]. D dimer is a potentially useful diagnostic tool prior to imagining, false negative can occur especially in patients with isolated headache or prolonged duration of symptoms (>1 week) [40].
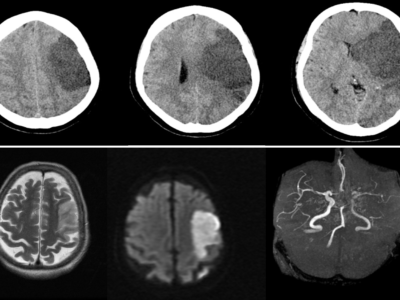
In one-quarter of patients, direct signs of CVT may be detected in plain CT, examples of finding are “dense triangle” a finding of a clot in the superior sagittal sinus, “ empty delta sign” (Fig. 1) for a no opacified thrombus surrounded by a collateral vein of the sinus wall after contrast injection, another sign is the “cord sign” (Fig. 2) indicated thrombosis of cortical or deep veins. Increased attenuation of the occluded sinus [41]. Nevertheless, these signs are less common in subacute/chronic cases and nonspecific. . CT venography is a sensitive technique for the detection of low flow, and demonstrating filling defects in the dural sinuses and veins can confirm the diagnosis of CVT. This is particularly useful in patients with contraindication to perform MRI or with limited access to MRI. However, the diagnostic yield of CT venography alone is limited by the presence of several anatomic variants that may mimic sinus thrombosis like sinus atresia or hypoplasia, asymmetrical sinus drainage, normal sinus filling defects related to prominent arachnoid granulations, or intrasinus septa, and the difficulties in detecting cortical veins thrombosis [18], [35].
![“Empty Delta sign” Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 4408 [36]](https://neuropedia.net/wp-content/uploads/2022/02/cerebral-vein-thrombosis-300x281.jpg)
Figure. 1 “Empty Delta sign” Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 4408 [36]
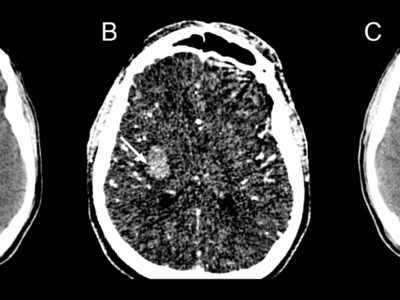
![“cord sign” Case courtesy of Dr Hani Makky Al Salam, Radiopaedia.org, rID: 13621 [37]](https://neuropedia.net/wp-content/uploads/2022/02/dural-sinus-thrombosis-and-venous-infarction-300x290.jpeg)
Figure. 2 “cord sign” Case courtesy of Dr Hani Makky Al Salam, Radiopaedia.org, rID: 13621 [37]
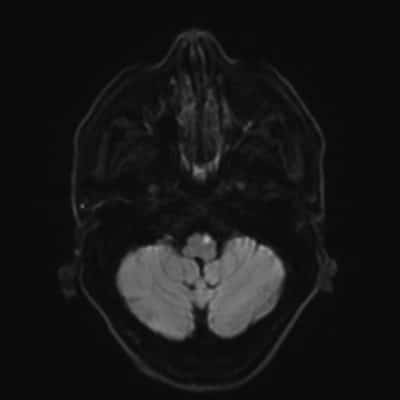
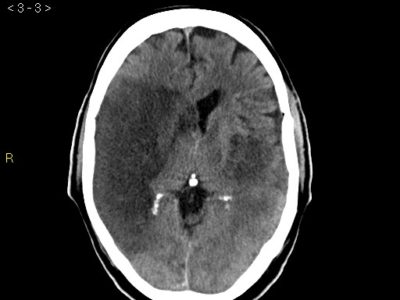
Small nontraumatic juxtacortical hemorrhages account for up to one fourth of intracerebral hemorrhages in patients with CVT and are associated with superior sagittal sinus occlusion. The brush sign (Fig. 3), an abnormally accentuated signal drop of subependymal and deep medullary veins in paramagnetic sensitive MR sequences, is occasionally found in CVT, especially in patients with thrombosis of the deep venous system or straight sinus, this sign likely represents increased deoxyhemoglobin and congestion of the deep veins and is associated with a clinical presentation with focal signs, more extensive thrombosis, and ipsilateral parenchymal brain lesion [42].
![figure. 3 “Brush sign” (a, b, T2*-weighted imaging) in a patient with thrombosis of the superior sagittal sinus, torcula, straight sinus, deep venous system, and left lateral sinus [4].](https://neuropedia.net/wp-content/uploads/2022/02/Brush-sign-a-b-T2-weighted-imaging-in-a-patient-with-thrombosis-of-the-superior_W640-300x183.jpg)
figure. 3 “Brush sign” (a, b, T2*-weighted imaging) in a patient with thrombosis of the superior sagittal sinus, torcula, straight sinus, deep venous system, and left lateral sinus [4].
Treatment
The European Stroke Organization along with the European Academy of Neurology issued a comprehensive guideline for the management of CVT [34]. The guideline followed the GRADE system, which increases objectivity, decreases bias, and makes the recommendations simpler and clearer. The recommendations are viewed in Table 2.
![Table 2. Recommendation of management of cerebral venous thrombosis [34]](https://neuropedia.net/wp-content/uploads/2022/02/CVT-295x300.png)
Table 2. Recommendation of management of cerebral venous thrombosis [34].
Patients with large hemispheric lesions, hemorrhagic, and impending herniation should be offered decompressive neurosurgery, or in general hemicraniectomy. No randomized controlled trial (RCT) of decompressive hemicraniectomy was specifically performed in CVT, but such a trial is unlikely to be feasible and will be considered unethical. In retrospective series, the vital and functional outcomes of decompressive craniectomy in CVT are much better than in ischemic or hemorrhagic stroke [47].
Endovascular acute treatment of CVT yet remains unproven as a therapy option. The US Guidelines state that endovascular treatment may be considered for patients who are comatose or who deteriorate despite starting them on anticoagulation and have no parenchymal lesions [48][49]. Due to the very low quality of available evidence, the European guidelines do not make any recommendations for using endovascular treatment in acute CVT patients with a pretreatment low risk of poor outcome [34]. A systematic review of case series of more than 3 CVT cases treated with mechanical thrombectomy, 40% of who had encephalopathy of coma, reported mortality of 14%, worsening or new intracranial hemorrhage in 9%, complete recanalization in 69%, and complete recovery in 35%. Chemical thrombolysis with mechanical thrombectomy did not result in additional harm nor benefit, in comparison with other techniques [50]. However, without a control group, no solid conclusions on the efficacy and safety of thrombectomy can be inferred. Two studies provided important evidence against the usage of endovascular thrombectomy or thrombolysis in acute CVT. The randomized controlled trial of thrombolysis or anticoagulation for severe acute CVT (TO-ACT) [51] was ended prematurely for futility. Of the 67 randomized patients, no difference was noted in clinical outcomes between those allocated to anticoagulation and endovascular treatment.
After initiating parenteral anticoagulation, oral anticoagulants like vitamin K antagonists should be used for at least 3–12 months, or for more prolonged periods in patients with genetic (e.g., protein C, S, and antithrombin deficiencies) or acquired (antiphospholipid syndrome, active cancer) prothrombotic conditions with a high risk of venous thrombosis recurrence [4]. The European Guidelines [34] recommend against the use of direct oral anticoagulants like factor Xa or thrombin inhibitors for the treatment of CVT, because of the low level of the available evidence, consisting of small case series without controls. Recently, a randomized controlled trial (RE- SPECT CVT) comparing the efficacy and safety of dabigatran 150 mg, bid, versus dose-adjusted warfarin (INR 2–3) for 6 months was completed, after allocating 60 CVT patients to each treatment arm. Results are waiting for publication [44]. The AHA/ASA guidelines recommend anticoagulation for 3–6 months in provoked CVT, 6–12 months in unprovoked CVT, and possibly lifelong anticoagulation in recurrent CVT, CVT followed by venous thromboembolism or CVT associated with severe thrombophilias.[52]
Outcome
Large CVT series confirmed that nowadays, an acute CVT showed low death rates about (0 to 2%) [17][48]. An important cause of death in acute CVT, but is much less frequent than seen after deep venous thrombosis of the limbs (1.4% vs. 6.6%, HR 0.26) [53]. In the last years, there was interest in knowing the subtle consequences of CVT in patients who recovered completely. About 1/3 of CVT patients did not return to paid work this was more frequently seen in females and in patients with parenchymal lesions [54]. In CVT patients with bilateral vision 10/10 and normal fundoscopic finding and visual field tests, optical coherence tomography demonstrated major axonal loss [55]. Male gender, myeloproliferative disorders, factor V Leiden mutation but not persistent venous occlusion were confirmed as risk factors for the recurrence of venous thrombotic events after a CVT event [56][57][58]. In a South-American cohort study [58], recurrence of venous thrombotic events after CVT was lower (1.6/100 patients-years) than in previous studies (~4/ 100), but the mean age was 30 years, and most CVT patients were females using oral contraceptives or pregnant, without associated prothrombotic conditions. Therefore, they recognized a lower risk of further venous thrombotic events.
Recanalization
In patients on anticoagulants, the rate of venous recanalization in the follow-up was found around 85% [59]. Analyzing the available cohort studies indicates that this process predominantly occurs in the first few months after CVT, although it can take up to 1 year. An association between the worse functional outcome and the lack of venous recanalization has been recently shown in a meta-analysis of CVT patients treated with anticoagulation [59]. More specifically, recanalization occurrence was related to a 3.3-fold increase in the odds of complete recovery. However, information on the temporal profile of recanalization was limited, hindering further conclusions on a possible critical time window for venous recanalization. Evidence on whether persistent occlusion increases the risk of recurrent CVT is even scanter, although an association has been shown in the pediatric population [51].
Follow-up imaging to establish the degree of recanalization can be useful to support or exclude the diagnosis of recurrent CVT in patients who have to worsen complaints, such as headache, during follow-up. Previous documentation of recanalization facilitates the diagnostic workup if patients with suspected recurrence.
Conclusions
Case-control studies are required to establish the association between several risk factors and CVT. Ideally, these studies should be performed in countries and centers with diverse health care conditions, systems, and available resources. Given the low prevalence of CVT and the ubiquity of some of its presenting symptoms like headache, cost-effective diagnostic studies of different imaging modalities and their combinations to identify CVT are desirable. The appropriateness of screening for acute CVT with some rare predisposing conditions must consider not only to reach a diagnosis but also patient-centered outcomes whether performing screening prevents death, leads to a better functional outcome, or decreases psychological distress. In the near future, results of the treatment trials on endovascular thrombolysis/thrombectomy (To- ACT), dabigatran (RE-SPECT CVT), and duration of anticoagulation (EXtending oral antiCOAgulation treatment after acute Cerebral Vein Thrombosis) will significantly increase the level of evidence that currently supports the management of CVT. [4]