Article topic: Low grade glioma
By: Shoroug Naser Al Hamdan
Key word: Low grade glioma ,grade II brain tumor .
Scientific editing : Dr.Omar Jbara
Linguistic editing : Philip Sweidan
- DEFINITION
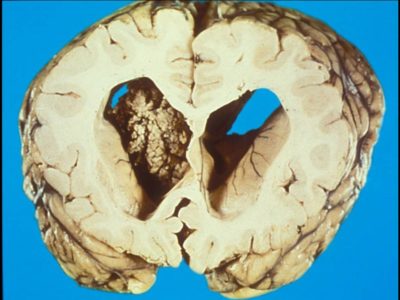
The term low-grade glioma (LGG) refers to tumors classified by the World Health Organization (WHO) as grades I and grade II, including oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas [1].
WHO grade I LGGs are truly benign tumors that can be treated with surgical removal . On the other hand, WHO grade II LGGs are diffuse and infiltrative intracerebral lesions rarely curable , [2] they are slowly progressing conditions that have the susceptibility for malignant transformation and almost invariably progress to a high-grade glioma[2][3] .Low grade glioma is the most common benign brain tumors in children.
- Epidemiology
The predicted incidence of grade II astrocytomas, oligodendrogliomas and mixed gliomas in the United States is 51%, 25%, and 20%, respectively, per 100,000 per year. [5] The Low grade gliomas occur more than twice as frequently in whites as in blacks .The peak incidence for astrocytomas is between 30 and 40 years of age, the peak incidence for oligodendrogliomas is slightly older (40 to 45 years of age)[5]. Low-grade gliomas are slightly more common in males.[4]
The risk factors for the development of low-grade gliomas are poorly understood. The only recognized environmental risk factor is distant history of ionizing radiation, such as in long-term survivors of childhood leukemia.[4] A history of allergies or asthma is somewhat protective against gliomas , perhaps suggesting a role for immune surveillance [4]. Well-defined inherited tumor predisposition syndromes ( neurofibromatosis type 1, Li-Fraumeni cancer syndrome, Lynch syndrome, Ollier disease, Maffucci syndrome, and melanoma neural system tumor syndrome) account for a minimal proportion of cases, but the 5% to 10% of patients with glioma with a positive family history for glioma and studies demonstrating a twofold glioma risk in first-degree relatives of patients with glioma pointing to other complex hereditary factors [4].
- Clinical features
Patients with low grade gliomas are usually younger than that for anaplastic gliomas or glioblastomas[4]. Although it is possible to see a newly diagnosed patient older than 60 years of age, the typical patient ranges nearly in age from the late twenties to the mid forties [4]. Most patients present with seizures, sometimes generalized tonic-clonic, but not uncommonly with a history of unrecognized partial seizures for several months or more [4]. Seizures are especially common with oligodendroglial histology, perhaps because oligodendrogliomas tend to invade the cortex [4].
Relatively it is uncommon for low-grade gliomas to present with fixed focal deficits, they tend to infiltrate rather than compress or destroy eloquent brain parenchyma [4]. Neuroimaging is typically critical for diagnosis of low-grade glioma[4]. More than 95% of these tumors are supratentorial. [6] Harshly equally split between frontal and temporal locations with very few occipital lobe tumors[4]. On CT, tumors in general show low attenuation [4]. Approximately 20% of low-grade gliomas establish calcification on CT, particularly oligodendrogliomas.[4] One-fourth of low-grade gliomas have several contrast enhancement on CT. [4]
This is usually patchy as opposed to ring enhancing [4]. The epicenter of low grade gliomas in general is in the white matter, but tumors with an oligodendroglial component tend to infiltrate and sometimes expand to the cortex [4]. MRI delineates tumors more clearly than CT, it usually shows low-grade gliomas as T1 hypointense and T2 fluid-attenuated inversion recovery (FLAIR) hyperintense; MRI might be more sensitive to small amounts of enhancement than CT.[4] Susceptibility weighted imaging (SWI) sequences can show calcification or, less commonly, hemorrhage; both of them are more common with oligodendrogliomas than astrocytomas.[4] Positron emission tomography (PET) scanning, diffusion and perfusion MRI and 1H magnetic resonance spectroscopy are usually of value in differentiating low-grade gliomas from non-neoplastic lesions and high-grade gliomas but are not routinely performed .[7]
Magnetic resonance spectroscopy for 2-hydroxyglutarate, which is elevated in IDH-mutant low-grade gliomas, has protruded as a research tool; technical refinements may eventually permit its use to follow low-grade glioma growth and response to therapy.[8]
- Pathogenesis and etiology
Low grade glioma (LGG) and secondary glioblastoma, which originates from a preexisting low grade glioma (LGG), were shown to harbor characteristic, neomorphic, heterozygous mutations in Isocitrate Dehydrogenase 1 (IDH1).[9]This cytosolic, NADP+ dependent enzyme physiologically forms a homodimer and patriciate in the conversion of isocitrate to α-ketoglutarate (α-KG), which can be used for multiple cellular functions.[10] Additionally, low grade glioma (LGGs) lacking a mutation in IDH1 may have an analogous mutation in IDH2, a mitochondrial enzyme that also produces α-KG, firstly for the TCA cycle.[11] .IDH mutations were found in >80% of tumors histologically classified as LGG.[9][17]
Further analysis of genomic sequencing revealed the being of two distinct molecular subclasses within LGG (Fig. 1).[18] The larger of these subclasses is characterized by inactivating mutations in the tumor suppressor gene TP53 and the chromatin remodeling enzyme ATRX (Fig. 1A,B).[19][20] The Tumors which contain this set of mutations correspond to astrocytoma, as identified by histopathology [21].The smaller subclass is characterized by the codeletion of chromosome 1p/19q and promoter mutations in telomerase reverse transcriptase (TERT) (Fig. 1B)[21].This latter group of tumors responsible for oligodendroglioma[18].
IDH mutant and P53/ATRX mutated (astrocytic) low grade glioma has a more aggressive clinical course[21]. While IDH mutant1p/19q co-deleted glioma (oligodendroglioma) has a less aggressive behavior, significant neurological decay and mortality does occur.[22][23].
All this classification neglects the small percentage of histopathologically graded low grade gliomas (LGGs) which do not exhibit a mutation in IDH1/2[18]. It has been suggested that these IDH wild-type gliomas behave like glioblastoma[18]. Indeed, if one looks for markers of IDH wild-type glioblastoma (EGFR amplification, H3F3A K27M or TERT promoter mutation), they can be found in half of these patients [23] These patients survive about 1.23 years, comparable to glioblastoma[12]. However, the other half of IDH wild type low grade gliomas don’t have these mutations and have a clinical course resembling more closely that of LGG with survival of more than 7 years.[23][24]
The most recent update to the World Health Organization guidelines on the classification of CNS tumors has incorporated the molecular features discussed above into the classification of adult low-grade glioma[18]. Histopathology remains critical in first diagnosing a tumor as a diffuse glioma and then in determining tumor grade [12]. If a tumor is determined to be a diffuse glioma and grade II or III, then molecular diagnostics command further classifications [12].
Mutation in IDH1/2 in a combination with loss of ATRX andTP53 results in the diagnosis of a diffuse astrocytoma or anaplastic astrocytoma, grade II and grade III [25]. If IDH mutations are found in combination with chromosomal1p/19q co-deletion then the diagnosis of oligodendroglioma or anaplastic oligodendroglioma is confirmed [25].
The diagnosis of oligoastrocytoma, previously defined as a tumor with both astrocytic and oligodendroglial histology, still in the updated guidelines but is much less common by the new definition [12]. Oligoastrocytomas now must have distinct tumor cell populations with the molecular features of both astrocytoma and oligodendroglioma, but these are exceedingly rare [26]. Finally, tumors which are histologically grade II or III and don’t have mutations in the previously discussed genes are grouped by histology into IDH wild-type astrocytoma or oligodendroglioma not otherwise specified (NOS) [25].
- Symptoms
Individuals with LGG cope with the burden of long-term symptoms and the associated challenges created by those outstanding symptoms [27]. [28] almost all patients with LGG are able to care for themselves, less than half of patients were able to perform unrestricted, normal activities of daily living and 45% reported low overall [27] patients with LGG reported more fatigue, cognitive impairment, speech difficulties, pain, sleep problems, and mood disturbances [28].
Symptoms of Low Grade Glioma depend on the size, the location of the tumor, rate of growth and consequences of increased intracranial pressure [29].
The majority of research on symptoms impacting the Quality of life (QoL) of glioma populations has been focuses on fatigue, sleep, pain, seizures, cognitive impairment, and mood disturbances [27]. For low grade glioma (LGG), a small study demonstrated that fatigue had the strongest relationship with Quality of life (QOL).[31] .
Seizures are the most common presenting symptom of low grade glioma (LGG), occurring in 65% to 85% of patients [32]. Uncontrolled seizures in patients with low grade glioma (LGG) have been reported in approximately 30% of patients, leading to cognitive fall and significant morbidity that can impact QoL. [32][33].
The management of seizures with anti-epileptic agents can also contribute to cognitive slowing and decrease QoL[27]. Mood disturbances, mostly described as anxiety, can occur in up to 60% of individuals with gliomas.[34][35].
Studies have reported that the depression at rates from 40% to 90% of patients in this population [34][35][36]. As such, a diagnosis of low grade glioma (LGG) is an important predictor of anxiety and depression.[34][35] In a cohort study of low grade glioma (LGG), depressive symptoms were noted to be the most important independent predictor of decreased QoL and possibly survival[36][37][38]. Psychological problems can exacerbate cognitive dysfunction (including memory)[39][40].
In 90% of long-term survivors of LGG, cognitive impairment has been found as the most serious challenge to cope with and adapt to post-treatment.[44][45] Even “symptom-free” survivors still have documented cognitive deficits on formal neurocognitive testing[46].
- Classifications
The 2016 WHO Classification of Tumors of the Central Nervous System [47] is an update of the previous classification from 2007 that includes, for the first time, molecular and genetic parameters, in addition to histopathologic features, to define and sub-categorize brain tumor entities [48]. This is a major breakthrough in pathological diagnosis that particularly restructures the diagnosis of diffuse gliomas[48].
Historically, it was not uncommon that mixed oligoastrocytic tumors were frequently diagnosed at some centers while they were rarely encountered at others [49]. The combination of histologic and genotypic parameters adds objectivity and inter-observer concordance, which results in improving the diagnostic accuracy [48].
The limiting factor for this new classification method is the actual availability and choice of genotyping or surrogate genotyping assays [48].
The Tumors which do not fit into the newly defined categories or those not being tested for molecular parameters are now classified under a NOS (Not Otherwise Specified) designation[49]. Diffuse gliomas are now grouped together according to the cell type of origin[48]. The reason is that they have same growth pattern, clinical behavior, genetic mutations and prognostic markers [48]. Diffuse gliomas now include WHO grade II and III astrocytomas and oligodendrogliomas, grade IV glioblastomas, midline gliomas and diffuse gliomas of the childhood [49]. Grade II diffuse astrocytomas and grade III anaplastic astrocytomas are now divided into three categories each: IDH-mutant (the great majority), IDH wild type (very rare) and NOS [48]. Nevertheless, the prognosis of IDH mutant cases appear to be more favorable than IDH-wild type for both grade II and grade III astrocytomas[53] .
Additional changes on the 2016 WHO classification include the deletion of two diffuse astrocytoma variants: protoplasmic astrocytoma and fibrillary astrocytoma (which largely overlaps with the standard diffuse astrocytoma) [48].
However, the gemistocytic astrocytoma remains an IDH mutant distinct variant [53]. The diagnosis of diffuse astrocytoma is depend on the confirmation of the mutant IDH status plus ATRX loss and TP53 mutation, both characteristic although not required for diagnosis [48].
Grade II oligodendrogliomas and grade III anaplastic oligodendrogliomas both exhibit IDH gene family mutations and 1p/19p codeletion, but no ATRX and TP53 mutations [53]. This fact statement an easy differentiation from astrocytomas[48].
However, the diagnosis of oligoastrocytoma is strongly discouraged by the 2016 WHO classification, and it is actually assigned to a NOS designation, since almost every mixed phenotypic tumor can be classified either as astrocytic or oligodendrocytic using genetic testing [54].
It is important to note that according to this new classification, grade II oligodendroglioma may progress to grade III oligodendroglioma but does not lead to grade IV glioblastoma, which always seems to derive from an astrocytic precursor [53].
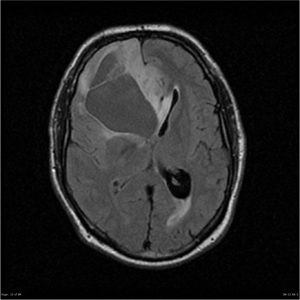
Diffuse astrocytoma, Case courtesy of Royal Melbourne Hospital,[55]
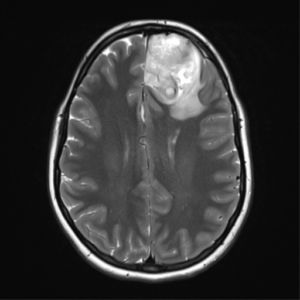
Case courtesy of Assoc Prof Frank Gaillard,[56]
Prognostic factors
A wide range of outcomes exists among patients with LGG [57]. Patient subsets can be identified with an average survival of as little as 2 years or greater than a dozen years [57]. Knowledge of prognostic factors is thus critical for therapeutic decision making and patient education [57].
Historically, neuro-oncologists depended upon readily available clinical, radiographic and neuropathologic features to determine prognosis [57]. Among clinical prognostic factors, age is powerful: younger patients do better than older patients, leading some studies to classify patients older than 40 years of age as high risk [57].
Presentation with isolated seizures is also favorable, possibly because of lead time bias; conversely, fixed neurologic deficits or mental status changes are prognostically adverse [57]. Tumor size also important, although whether preoperative or postoperative size is more critical remains uncertain [58][59]; similarly, involvement of the corpus callosum is a recognized adverse characteristic[60].
Neuropathologic assessments provide valuable prognostic information [60]. It has long been appreciated that median survival of oligodendrogliomas is nearly twice that of astrocytomas.[61] Newer molecular tests allow more refined prognoses and to what extent incorporation of such tests might reduce the importance of some aforementioned clinical factors is still unknown[60].
The first molecular test of prognostic value was 1p/19q codeletion status, which correlates to some extent with classic histology but avoids interpreter discrepancy; for example, one study found a median survival of 12 years in co-deleted LGG versus 8 years in non-codeleted tumors, [62] the danger ratio for overall survival for patients with 1p deletion was 0.59, similarly supporting the favorable prognosis of codeleted tumors [63].
The recently discovered IDH mutation present in most LGG has a similar favorable prognostic effect, 11 years [64] Classifying LGGs into three molecular groups (1p/19q-codeleted, IDH mutated but 1p/19q intact, and neither alteration) yields three distinct survival curves with median survivals of approximately 9, 6, and 2 years, respectively[65]. Additional molecular markers (e.g., TERT mutations) will certainly improve molecular prognostication in coming years [66].
- DIAGNOSTIC NEUROIMAGING FOR GLIOMAS
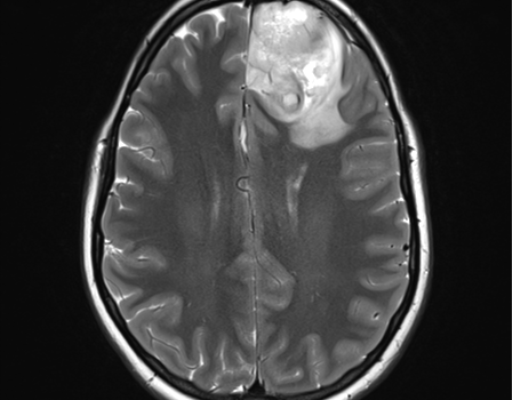
Magnetic resonance imaging (MRI) of low grade glioma demonstrates lesions that are isointense/hypointense on T1-weighted images, are homogeneously hyperintense on T2-weighted images and do not enhance with contrast administration [67].
Some of these characteristics are shared with high-grade gliomas (HGGs), with the exception of contrast enhancement, which is usually, but not always, seen in more aggressive grades of high grade glioma, [68] calcifications can be revealed in about 20% of lesions and appear as distinct hyperintense foci on T1-weighted images and hypointense foci on T2-weighted images[69].
Vasogenic edema and necrosis are not typical of Low grade gliomas , owing to their slow growth rate[67]. More sophisticated MRI techniques, such as MR spectroscopy, have been used to differentiate glioma grades and even to detect key Low grade glioma metabolic mutations, such as those of the isocitrate dehydrogenase1 (IDH1) gene[67].
These tumors were also graded with use of proton MR spectroscopy of metabolite ratios (choline/creatine, choline/N-acetylaspartate and N-acetylaspartate/creatine), which yielded significant differences between Low grade gliomas and high grade gliomas (P < .01); sensitivity, specificity, PPV, and NPV rates were 93.3%, 60.0%, 87.5% and 75%, respectively[70].
An increased choline-creatinine ratio on MR spectroscopy also gets along with heightened risk of transformation, with a sensitivity of 80% and a specificity of 94%.[71] Interestingly, work has now determined that proton MR spectroscopy can detect Low grade glioma -specific oncometabolites, such as 2-hydroxyglutarate, a metabolite of mutant IDH1.[72]
A prospective study of 27 patients shows that elevated choline and decreased glutathione values correlated with IDH1 mutation in gliomas[57]. Tumors with this mutation follow a better prognosis and may be responsive to certain chemotherapy regimens.[67]
Although IDH1 mutations are not exclusive to low grade gliomas, this strategy provides surgeons with a noninvasive prognostic marker.[67] Yet, metabolic imaging of low grade gliomas extends beyond the use of magnetic resonance and is increasingly explored with use of positron emission tomography (PET).[67]
Both fluorodeoxyglucose-PET (FDG-PET) and fluorothymidine-PET (FLT-PET) have been used to evaluate LGG metabolism and proliferation [67]. A prospective study of 60 patients with cerebral gliomas evaluated the ability of FDG-PET to differentiate low grade gliomas from HGGs [67]. The investigators mention a PPV of 97.3% and an NPV of 70.2% [73].
Additionally, a study of 25 patients with newly diagnosed or previously treated gliomas demonstrated that decreased FLT-PET uptake correlated with a lower Ki-67 index and the Low grade glioma classification [74].
Perfusion MRI is one of several new physiologic imaging modalities with promising utility for grading low grade gliomas[67]. A number of retrospective studies have indicated that relative cerebral blood volume (rCBV) can be used as a preoperative predictor of Low grade glioma histology [67]. The sensitivity and specificity of using rCBV for differentiating Low grade gliomas and HGGs is reportedly 80% to 95% and 94% to 96%, respectively.[67]
Additionally, a study of 69 patients demonstrated an inverse correlation between rCBV and time to progression or death, with an odds ratio of 1.87 (95% confidence interval [CI],(1.14-3.08).[75] This finding was supported by a subsequent study of 20 patients in whom increased rCBV was associated with rapid progression of Low grade gliomas[67]. Similarly, diffusion tensor imaging (DTI) is under investigation to differentiate LGGs and HGGs histologic appearances [67].
A retrospective study of 78 patients with gliomas found an inverse correlation between tumor grading and axial diffusivity (AD), radial diffusivity (RD) and the apparent diffusion coefficient (ADC)[76]. DTI analysis of 79 gliomas also explain a correlation with the tumor grade and was able to differentiate LGGs from HGGs with a sensitivity of 92% to 94% and a specificity of 53% to54%.[77]
These findings are supported by a retrospective study of 25 patients that explain specificity and sensitivity of 86% and 80%, respectively, for differentiating LGGs from HGGs with use of spherical isotropy coefficients (CP and CS) and fractional anisotropy (FA)[77]. Both rCBV and DTI are promising new modalities to better characterize tumor heterogeneity and prophesy regional transformation [67].
Functional MRI (fMRI) is based on the increasing in blood flow to local vasculature that accompanies neural and metabolic activities in the brain [67]. This increase results in a corresponding local reduction in deoxyhemoglobin because the increasing in blood flow occurs in the absence of a comparable increase in oxygen extraction [67]. Deoxyhemoglobin is used as an endogenous contrast enhancing agent and serves as the source of the signal during fMRI[67].
Functional MRI results can be consistent with those of electrophysiology, PET, cortical stimulation, and magnetoencephalography (MEG) and are mainly used to provide preoperative functional and structural information for neurosurgery [67].
Cortical stimulation, which still the gold standard for functional localization is based on local circuit disruption or activation and best identifies areas that are essential to language processing [67].
In contrast, fMRI is an activation-based method that identifies all regions of the brain that demonstrate activity related to a specific task, regardless of whether those areas are essential or supplementary[67].
Consequently, areas that show negative for language when cortical stimulation is used may still demonstrate fMRI activation, producing false-positive results[67]. Decreasing in specificity may also be expected because fMRI is a perfusion-based method and does not directly detect neuronal activity [67].
Magnetoencephalography (MEG) is also used for preoperative functional mapping[67]. Magnetoencephalography (MEG) imaging reconstructs the spatiotemporal dynamics of brain sources from magnetoencephalographic data.[67] When co-registered with an anatomical scan, such as a high-resolution MR image, magnetoencephalography (MEG) imaging can be integrated into a neuronavigation system and provide intraoperative guidance to the surgical team. [67]
Preoperative MEG maps of the motor cortex have been validated compared to intraoperative direct cortical stimulation (DCS) maps and are a useful modality for preoperative localization of this region [67]. Relative to fMRI and PET, MEG has the advantage of higher temporal resolution because it measures direct neuronal activation instead of indirect hemodynamic change. [67]
Previous studies have also suggested that MEG is more accurate than fMRI in identifying functional cortices that have been distorted by a nearby tumor.[67] Overall, MEG is a robust and credible functional imaging modality that is now used to identify the cortical location of motor and sensory pathways. [67]
Integrating MEG data with DTI information into a neuronavigational workstation directs the neurosurgeon toward potential functional sites that can be intraoperatively assured with stimulation mapping [67]. Magnetic source imaging (MSI),which is based on the magnetoencephalographic detection of the late neuromagnetic field extracted by simple speech sounds[78] is another mapping technique that can be useful for mapping the somatosensory cortex and determining hemispheric dominance, and it may serve as a replacement of the Wada test.[67]
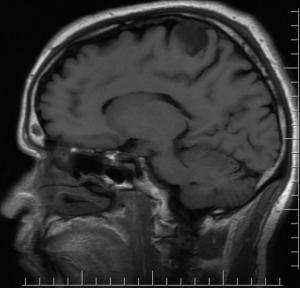
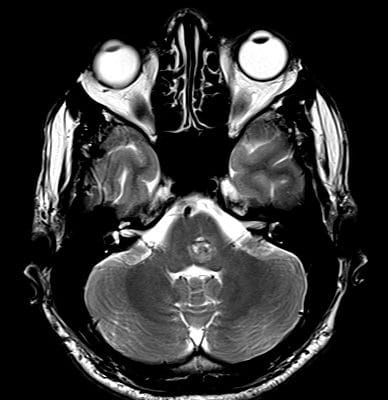
Sagittal T1, low grade glioma ,Case courtesy of Dr Prashant Mudgal,[79]
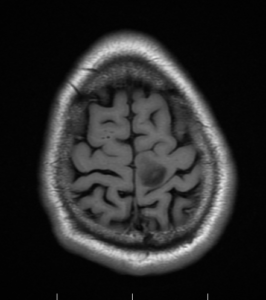
Axial T1, low grade glioma , Case courtesy of Dr Prashant
Mudgal [80]
- Treatment
Low grade gliomas (LGGs) are treated depending on where they are located, They are commonly treated with surgery, which is sometimes followed by chemotherapy and radiation, when surgery is not possible, other options can be considered, including observation, chemotherapy or radiation.
- .SURGICAL DECISION MAKING
After MRI shows an abnormality compatible with a low-grade glioma, the question becomes whether to intervene surgically and, if so, whether to biopsy or to perform maximal safe resection of the tumor [60]. When a patient has neurologic deficits or significant mass effect, the need to intervene is necessary [60].
However, the patient with an incidentally revealed lesion or well-controlled seizures presents a different problem[60]. Currently, no compulsory evidence exists that early intervention improves outcome over observation, with surgery when the lesion grows, supporting a watch and wait approach in some cases[60]. Countering this philosophy is the reality that (LGG) grow continuously, so detection of growth is simply a matter of time [61].
Moreover, approximately 50% of anaplastic gliomas, tumors that should be treated promptly after diagnosis do not enhance and thus may resemble (LGG) on MRI [60]. Patient preference and prognostic factors, including age, tumor size and location must be considered in the approach to this decision [60]. If observation is selected, patients should be carefully followed with serial imaging compared to the initial abnormal scan to ensure that large changes in tumor size that might preclude effective interventions, such as debulking, are not missed [60]. Once the patient and neurosurgeon have decided to pursue eventual diagnosis, the next question is whether to resect or biopsy the tumor [60].
Level one, evidence is lacking and unlikely ever to be available [60]. A retrospective population-based parallel cohort study in Norway compared patients who underwent maximal safe resection to patients who underwent biopsy followed by observation until progression in both groups demonstrated improved overall survival in the first group. [62]
A large series of 216 patients with (LGG) who underwent resection found that extent of resection correlated significantly with overall survival, with a 5-year overall survival of 97% with an extent of resection greater than 90% but only 76% with an extent of resection less than 90%.[63] Also, a study of 170 patients from Johns Hopkins reported 5-year overall survival of 95% with gross total resection compared to 70% with subtotal resection [64]. Admittedly, bias undoubtedly found in terms of which patients undergo aggressive resection versus limited resection or biopsy. [60]
Some studies have shown that although extensive surgery predicts improved overall survival on univariate analysis, this finding is lost when data are controlled for other prognostic factors [61]. Admittedly advantage of debulking is its improved diagnostic accuracy: needle biopsies have a substantial misdiagnosis rate as they may undersample a heterogeneous tumor with foci of anaplastic glioma. [65][66]
For these reasons, most authorities favor maximal safe resection, with the understanding that tumors involving the corticospinal pathway, speech areas and other eloquent regions may not be docile to aggressive surgery.[60] Functional MRI (fMRI), intraoperative mapping [67], awake craniotomy and intraoperative MRI are techniques that help the surgeon in the quest for maximal safe resection [68].
- ROLE OF RADIATION AND CHEMOTHERAPY
The optimal management of (LGG) remains one of the most contentious areas in neuro-oncology, with questions about the timing and sequence of radiation and chemotherapy largely unresolved [60]. One issue is if patients who have had excellent resections may be observed without additional treatment [60].
A prospective study of observation in patients younger than 40 years of age who had undergone gross total resection of a (LGG) found that slightly more than 50% recurred within 5 years of surgery [81].
Thus, while initial observation is a reasonable postoperative option in low-risk patients, they require long-term surveillance imaging, and the follow-up scans should be compared to the early postoperative MRIs instead of the most recent scan so that tumor progression can be accurately detected [60]. Observation is sometimes selected following aggressive debulking in higher-risk patients, but the likelihood of early recurrence is correspondingly increased [60]. Once the decision is made for treatment, options include fractionated radiation therapy, chemotherapy, or the combination of the two modalities [60]. Fractionated external beam radiation therapy has been the initial treatment. [60]
As doses from 45 Gy to 64 Gy in 1.8 Gy to 2 Gy daily fractions achieve similar tumor control, a dose of 50 Gy to 54 Gy to the tumor and margins over 5 to 6 weeks has become standard[82][83]. Nearly 30% of patients experience significant tumor shrinkage.[60]
It is difficult to quantifying the survival benefit of radiation, since a study that proscribed radiation would be unethical [60]. A phase 3 European study randomly assigned patients with (LGG) to early radiation versus observation, with radiation permitted for tumor progression [60].
Early radiation prolonged progression-free survival by 2 years, but overall survival was the same between the groups. [84] This study can be interpreted to support either early treatment or observation depending on one’s viewpoint [60]. It is difficult to Determine of tumor activity is sometimes challenging after radiation therapy, as the treatment itself often produces delayed white matter changes that may resemble progressive tumor[60]. Recently devised criteria for (LGG) tumor response provide some guidelines for subsequent scan interpretation [85]
Radiation therapy almost always produces some short-term fatigue and studies of cognitive functions often show at least temporary decline in memory [60]. Since patients with low grade gliomas not uncommonly experience a slow decline in performance status over years following diagnosis, much of this has been blamed on radiation therapy [60].
Recent studies suggest that in the first several years after radiation, most cognitive decline is referred to the tumor itself or concomitant medications such as anticonvulsants [86]. Nonetheless, with longer follow-up, it is clear that even modern radiation techniques and dosing are associated with measurable cognitive decline in long-term survivors with (LGGs) [87].
The recognition in the 1990s that anaplastic oligodendrogliomas were chemosensitive, coupled with the hope of deferring cognitive risks of radiation, created interest in exploring chemotherapy as the initial antineoplastic therapy in (LGGs) [60].
Studies were done about the combination of procarbazine, thenitrosourea CCNU (lomustine), andvincristine (PCV) used for anaplastic oligodendrogliomas; with the emergence of temozolomide and it has improved side effect profile, attention shifted to this agent[60]. Small phase 2 chemotherapy studies have demonstrated that tumor response rates close to those of radiation therapy, with tumor stabilization ranging from 3 to 5 years [60].
These encouraging results led to a large phase 3 trial (European Organization for Research and Treatment of Cancer [EORTC]22033-26033) that randomly assigned patients with high-risk LGG (older than 40 years of age, symptomatic, or with tumors growing under observation) to either 1 year of temozolomide or standard radiationtherapy [60].
Initial results, with median follow-up of 4 years, were published in 2016.35 For co-deleted (LGGs), median progression-free survival was similar with temozolomide and radiation (55.0 versus 61.6 months)[60]. On the other hand, for IDH-mutant non–co-deleted tumors, progression-free survival was markedly inferior with temozolomide (36.0 versus 55.4 months =.004), suggesting that this approach is not justified in this tumor profile [60].
Given the ultimately fatal outcome of low grade gliomas and the relative successes combining radiation with chemotherapy in higher-grade gliomas, it is unsurprising that combined chemotherapy and radiation approaches for low-grade glioma are being studied [60]. Preliminary results of a relatively large single-arm phase 2 study (Radiation Therapy Oncology Group [RTOG] 0424) combining radiation therapy with temozolomide suggest improved survival compared to a historic control group treated with radiation therapy alone,[88] and a phase 3 trial randomly assigning high-risk patients with LGG to radiation with or without temozolomide (Eastern Cooperative Oncology Group [ECOG] E3F05) was launched[60].
Recently updated results incorporating long-term follow-up from an old low-grade glioma trial suggest that radiation therapy alone is inadequate therapy for low-grade gliomas[60]. In 1998, a phase 3 trial that randomly assigned patients with LGGs to radiation with or without subsequent PCV were launched [60]. The original publication of trial results in 2012 did not demonstrate an overall survival advantage with the addition of chemotherapy despite an improvement in progression-free survival [89].
The tails on the overall survival curve were diverging [60].With 6 additional years of follow-up, [90] a statistically worthy benefit in overall survival favoring the PCV arm (13.3versus 7.8 years) was seen [60]. Unfortunate, inadequate tumor tissue precluded breaking down these results by IDH and 1p/19q status; similar studies in anaplastic gliomas demonstrated that the bulk of benefit from adjuvant PCV was in codeleted tumors [60]. Nonetheless, patients with LGGs requiring treatment with radiation should strongly be considered for combined chemotherapy and radiation [60].
Whether temozolomide, a better-tolerated oral agent, can substitute for the three-drug PCV combination remains unknown and will presumably require a prospective randomized trial to address; the ongoing radiation therapy with concomitant and adjuvant Temozolomide versus radiation therapy with adjuvant PCV chemotherapy in patients with anaplastic glioma or LGG (CODEL) trial (NCT00887146) comparing temozolomide to PCV in codeleted anaplastic gliomas was recently amended to include codeleted low grade gliomas and may help address this.[60] An important unresolved issue is how an initial approach of using chemotherapy alone to defer radiation therapy followed by using combined chemotherapy and radiation at progression compares to initial combined chemotherapy and radiation, both in terms of overall survival and quality of survival from a cognitive and quality-of-life perspective[60].
It is possible that the early use of alkylating agent chemotherapy could reduce the effectiveness of further chemotherapy added to radiation therapy at tumor progression or lead to the development of additional harmful mutations in the tumor [91]. How the heterogeneous group of patients with IDH-wild type grade II gliomas should be treated is uncertain; many including most older patients will have a clinical course consistent with glioblastoma and presumably should be treated [60]. Regardless of initial treatment, LGGs eventually resume growing [60]. The majority will then develop increasing enhancement and if operated on again, will be found to have transformed into higher-grade gliomas[60].
Although this phenomenon is often called malignant transformation, that term overlooks the fact that LGGs themselves are low-grade malignancies [60]. At progression, patients who firstly received radiation therapy are candidates for chemotherapy and vice versa [60].
The Patients who progress after receiving temozolomide may receive a nitrosourea alone or as part of PCV, whereas those progressing after nitrosoureas generally go on to temozolomide[60]. Bevacizumab, an active agent for glioblastoma, was studied in recurrent/progressive LGGs in a 2016 randomized phase 2 trial; the addition of bevacizumab to temozolomide did not appear to afford benefit in contrast-enhancing recurrent tumors, which is perhaps unsurprising since LGGs are not vascular endothelial growth factor (VEGF) Y driven tumors [92]. LGGs are ultimately fatal, although clinical trials targeting the mutant IDH protein are under way.[60]
- Conclusion
Innovations within the diagnostic, therapeutic, and molecular domains are intertwining and helping us understand and treat low grade gliomas (LGGs) more effectively.
Prognostic factors derived from genetic analysis and clinical characteristics allow us to apply patients into proper treatment groups to maximize therapeutic benefit. Stereotactic biopsies provide information to clinician regarding pathologic grade, which is used to give the patient a prognosis and suggest more effective treatment options. Additionally, large retrospective multi-institutional studies have shown that maximizing the extent of resection can delay recurrence and improve the time to transformation. However, this approach must be balanced with preservation of neurologic function, which can be better by using intraoperative mapping.
Evidence also shows that chemotherapy with radiation therapy may prolong Progression free survival and Overall survical. Many challenges have been overcome, in terms of providing an accurate prognosis and assigning treatment regimens, but our findings are limited because low grade gliomas (LGGs) are not homogeneous and small genetic changes can significantly affect outcomes.
Future clinical trials that classify patients according to novel prognostic factors maybe aid increasing patient-specific treatment plans with better outcomes.
- External links
Case in low grade glioma(https://www.astro.org/ASTRO/media/ASTRO/AffiliatePages/arro/PDFs/ARROCase_LowGradeGlioma.pdf).