Topic: The ASPECTS score; Alberta Stroke Program Early CT Score
Author: Dania Raid Ismail Abuhashish
Editor: Shaden Tashtoush, Ihda Bani Khalaf
Reviewer: Ethar Hazaimeh
Keywords: ASPECTS, pc-ASPECTS, NCCT, MCA
Introduction
Originally introduced in 2000, the Alberta Stroke Program Early CT Score (ASPECTS) is a 10-point scoring system used to quantify early ischemic changes on non-contrast computed tomography (NCCT) in patients with acute ischemic stroke. The ischemic changes are viewed on NCCT scans for patients who may need endovascular therapy, such as for MCA (Middle Cerebral Artery) strokes. Moreover, it can be adjusted for posterior circulation, otherwise known as pc-ASP6ECTS [1].
ASPECTS divides the MCA territory into 10 predefined regions: six cortical regions (M1–M6) and four subcortical structures—the caudate, lentiform nucleus (putamen and globus pallidus), internal capsule (posterior limb), and the insular cortex [2].
Each region with evidence of early ischemic change reduces the ASPECTS by one point from a maximum of 10; consequently, this results in a linear decrease in score as lesion burden increases [2]. Moreover, the Alberta Stroke Program Early CT Score reliably predicts functional outcome in ischemic stroke and helps identify the effectiveness of endovascular and thrombolytic treatments [1].
Clinical Use of ASPECTS in Stroke Evaluation
ASPECTS is effective in monitoring early blood deficiency in the brain. Consequently, by categorizing the severity of ischemic progression, ASPECTS provides estimates of the size of cerebral infarction [2].
Furthermore, ASPECTS aids in guiding treatment decisions, evaluating early ischemic progression, and estimating infarct size. While ASPECTS was primarily designed for MCA strokes, adaptations such as pc-ASPECTS have extended its use to posterior circulation strokes [2].
ASPECTS Regional Scoring System
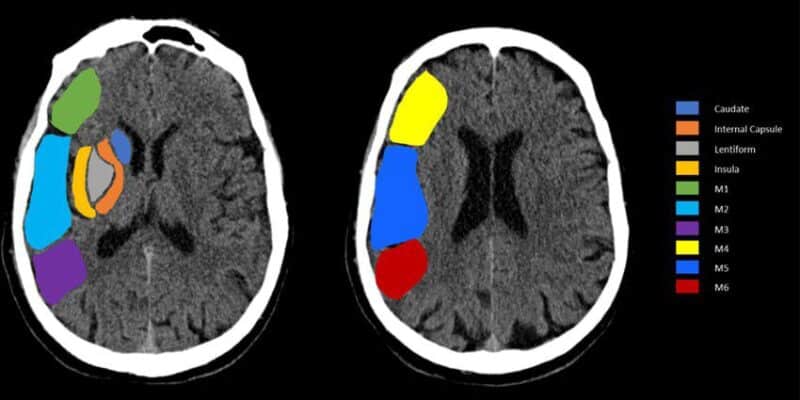
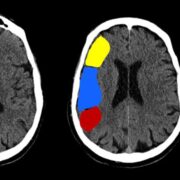
There are 10 brain regions ASPECTS monitors (Figure 1). The ganglionic areas of ASPECTS are the caudate nucleus, putamen, insular cortex, and internal capsule posterior limb. Meanwhile, the other segments are portions of the middle cerebral artery [2].
Regarding cortical M regions, they differ in terms of their position relative to the MCA cortex. There are six different M regions regarded in the ASPECTS score [2].
- M1 is anterior to the MCA cortex, whereas M2 is lateral to its insular ribbon.
- M3 is located in the posterior part of the MCA territory, typically involving the posterior parietal or posterior temporal regions.
- M4, M5, and M6 represent the superior cortical regions of the MCA territory—M4 is anterior, M5 is lateral, and M6 is posterior—positioned above M1, M2, and M3, respectively [2].
The scale is therefore measured based on the hypodensity of the stroke found in any of the aforementioned areas [2]. A point is deducted from the initial 10-point score for every region with an abnormality [2].
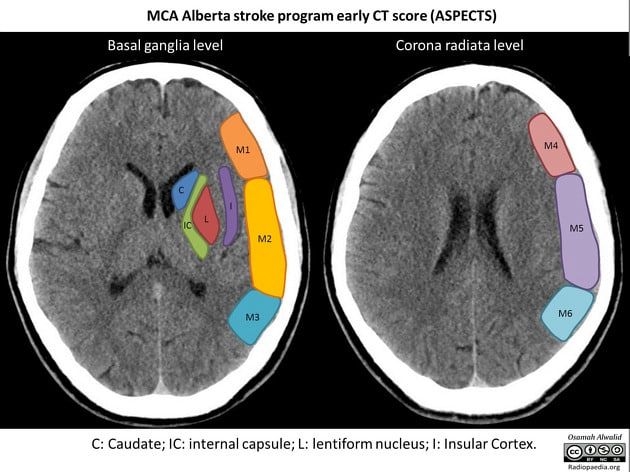
Figure 1: Illustration of the Alberta stroke program early CT score (ASPECTS) of the middle cerebral artery. [23]
Therapeutic Applications of ASPECTS
ASPECTS is a quantitative CT score with significant potential as an evaluation tool for stroke progression. By consistently tracking ischemic changes via ASPECTS, treatment can be adjusted appropriately. Specifically, the main uses of ASPECTS are determining the severity of middle cerebral artery strokes based on CT scans and predicting functional outcome after intravenous thrombolysis [3].
Initially, intravenous thrombolysis is used to treat acute ischemic stroke, such as tissue-type plasminogen activator (tPA) [4]. Subsequently, in acute ischemic stroke patients, intra-arterial treatment is effective if administered within 6 hours after symptom onset [5]. The ASPECTS score is reliable in predicting stroke patients who are unlikely to make a full recovery with thrombolytic treatment and serves as a guide in thrombolytic therapy [6].
Moreover, in 2015, Goyal et al. conducted a trial evaluating mechanical thrombectomy with 316 participants. ASPECTS was used for the primary outcome and in calculating the common odds ratio during patient exclusion. The primary outcome indicated that rapid EVT intervention was favorable (common odds ratio = 2.6 and 95% CI). Thus, ASPECTS was an important imaging measure in this assessment [7].
PC-ASPECTS in Posterior Circulation Stroke
Posterior circulation ASPECTS also uses a ten-point scale. However, unlike ASPECTS, any midbrain or pontine involvement reduces the score by two points (Figure 2). Whether the involvement is unilateral or bilateral, it is considered a two-point deduction [8].
Diagnosing posterior circulation strokes has been challenging despite NCCT and NIHSS. In 2021, Caruso et al. found a method to diagnose such strokes using pc-ASPECTS [9]. Specifically, their research tested ASPECTS’ prognostic value, particularly when used alongside CT perfusion imaging, in predicting functional outcomes in posterior circulation strokes [9].
Furthermore, pc ASPECTS can identify basilar artery occlusion functional outcomes. For example, Volker Puetz et al. concluded that pc-ASPECTS can predict patients with basilar artery occlusion with adverse functional outcomes despite recanalization [8].
Consequently, in basilar artery occlusion, patients with pc-ASPECTS ≥ 5 can benefit from endovascular therapy. Sang et al. (2021) validated pc-ASPECTS as a prognostic and treatment-guiding tool in BAO, showing that patients with higher scores had better functional outcomes following endovascular therapy [9].
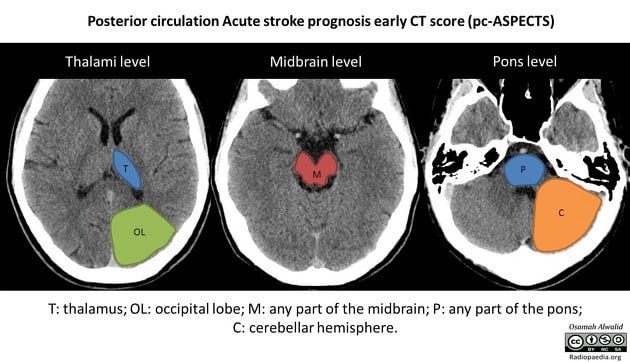
Figure 2: Illustration of the acute stroke prognosis early CT score (ASPECTS) of the posterior circulation. [24]
Early Detection and the Ability of ASPECTS
In 2020, Wolff et al. conducted a validation study using CT scans from the MR CLEAN trial cohort to assess ASPECTS in identifying early ischemic changes on NCCT scans. According to the study, a two-observer consensus served as the reference standard. The study reported an area under the curve (AUC) of 0.750–0.795, with a specificity of 0.89 and sensitivity ranging from 0.41 to 0.57 (n = 355) [11]. These findings. Therefore, support ASPECTS as a structured and reliable tool for detecting early ischemic changes, with acceptable interobserver agreement even among experienced radiologists [11].
Role of ASPECTS in Reperfusion Therapy
ASPECTS has demonstrated clinical value in predicting outcomes following intravenous thrombolytic therapy. In one study, baseline ASPECTS predicted rehabilitation outcomes in patients receiving intravenous alteplase. Pre-treatment CT scans were obtained from 203 patients with ischemic stroke [12].
Notably, approximately 75% of those with anterior circulation ischemia exhibited ischemic changes on imaging. Baseline ASPECTS scores were significantly associated with symptomatic intracerebral hemorrhage (p = 0.012) and functional outcomes (p < 0.001). Thus, ASPECTS demonstrated a sensitivity of 0.78 and specificity of 0.96. For predicting symptomatic intracerebral hemorrhage, the sensitivity was 0.90, and the specificity was 0.62. Taken together, these findings affirm ASPECTS as a practical prognostic tool in guiding thrombolytic therapy [12].
Comparison of the ⅓ MCA Rule and ASPECTS
It is paramount that techniques used in monitoring ischemic change have clearly defined conditions in which their performance is maximized, and the efficacy of endovascular thrombectomy in patients with acute ischemic stroke presenting 6 to 16 hours after symptom onset is investigated extensively [13].
Detecting acute MCA strokes has been challenging. Previously, physicians relied on the 1/3 MCA rule, which is a flawed estimate of MCA infarction [14]. Specifically, the rule states that if more than 1/3 of the MCA territory is involved in NCCT, there is an increased risk of hemorrhage with tPA therapy [15].
In 2003, Mak et al. evaluated the validity of the 1/3 MCA rule versus ASPECTS in detecting early ischemic changes (EIC) of acute stroke. The study used CT scans of patients with suspected acute ischemic strokes within hours of symptom onset. The study thresholds were: 1/3 MCA rule k=0.49 with PABAK=0.74, whereas ASPECTS ≥ 7 had k=0.34 with PABAK=0.44 [14].
The 1/3 MCA practice confirmed considerable early ischemic change in 11.4% of CT scans, whereas ASPECTS identified 19.4%. Although the 1/3 MCA rule was widely used within 6 hours of stroke onset, ASPECTS is now preferred because it detects significant EIC at a higher rate [14].
Furthermore, Kalafut et al. noted that the 1/3 MCA rule is valid when used by skilled neuroradiologists; otherwise, it may fail to identify early CT infarctions, putting patients at increased risk of hemorrhage after tPA treatment [15].
ASPECTS with NCCT, CBV, and PBV
CT perfusion (CTP) alongside ASPECTS has been beneficial in decision-making for acute ischemic stroke. A 2011 RCT observed detection of reversible ischemia on CTP ASPECTS, concluding that CTP ASPECTS can identify potentially recoverable ischemic brain tissue [16].
In addition, Aviv et al. compared CTP ASPECTS to NCCT ASPECTS outcomes. Qualitative CT perfusion ASPECTS is a sound indicator for early ischemic strokes and can guide AIS decision-making [17].
For example, in 2007, Aviv et al. evaluated 36 acute stroke cases within 3 hours of symptom onset, comparing NCCT ASPECTS and CT-CBV (cerebral blood volume) maps. ASPECTS demonstrated substantial interobserver agreement and, importantly, was a significant predictor of neurologic improvement (p = 0.02), highlighting CBV ASPECTS’ value alongside NCCT ASPECTS [18].
CBV ASPECTS is preferable to NCCT in radiological outcome prediction and is a favored predictor for identifying patients likely to make a notable neurological recovery [18].
While NCCT ASPECTS is the most commonly used due to accessibility, CBV-ASPECTS has superior reproducibility and predictive value, and both CBV and PBV ASPECTS may provide additional information on tissue viability in selected cases [18].
NCCT is also a practical exclusion tool for identifying patients likely to suffer ischemic damage from ineffective endovascular therapies [19].
A 2021 retrospective study assessed PBV maps in 37 patients with acute MCA M1 occlusion who underwent CT perfusion before mechanical thrombectomy. Matched-pair analysis compared CBV and NCCT scores [20]. NCCT ASPECTS had the highest w-kappa of 0.74, CBV ASPECTS = 0.63, and PBV ASPECTS = 0.53. In contrast, the study noted infarct overestimation in both CBV and PBV ASPECTS, mostly in PBV ASPECTS [20].
Modern Innovations and Refinements in ASPECTS
In 2021, Naganuma et al. assessed ASPECTS alongside a deep learning brain hemisphere comparison algorithm (3D-BHCA). Specifically, non-contrast CT data were collected from AIS and non-stroke patients. Region-based analysis thresholds were 0.96 accuracy, 0.97 specificity, and 0.8 sensitivity. Dichotomized ASPECTS > 5 analysis had higher specificity and accuracy [21].
Thus, 3D-BHCA enhances ASPECTS potential, making it comparable to assessments by stroke neurologists [21].
Accessibility
Both smartphones and medical monitors can accurately perform ASPECTS scoring, particularly when done by qualified radiologists [22]. ICC values > 0.74 with p<0.05 were found in a retrospective research [22].
Salazar et al. contended that radiologists who were more skilled at grading lesions, especially in the M2, insular cortex, and internal capsule, might improve ASPECTS ratings. As a result, this would decrease thrombolysis complications and increase efficiency. [22]











So proud of you and the incredible work you’ve done on this research.