Topic: REM Sleep Behavior Disorder (RBD)
Author: Udi Alkhazaleh
Editor: Abdel Rahman Bani Yassin, Ihda Bani Khalaf
Reviewer: Ethar Hazaimeh
Keywords: REM sleep behavior disorder (RBD), Parasomnias, Neurodegeneration, Parkinsonism, α-synuclein neurodegeneration
Overview
Parasomnias are a group of disorders characterized by abnormal movements, behavior, emotions, and/or perceptions that occur during specific sleep stages while falling asleep, during sleep (e.g., Rapid eye movement (REM) or Non-rapid eye movement (NREM) sleep), and during the sleep-wake transition phase.(1) RBD stands for REM sleep behavior disorder characterized by dream enactment due to loss of REM sleep atonia, which is described by physically acting out during sleep, especially if he is dreaming and starts taking his actions out to reality; in the meanwhile, the patient is unaware of what he has done.(2) Patients are unaware of their acts and do not remember what they have done because it occurs in REM sleep, when the brain is in a dream state and consciousness is not present. Thus, this condition is commonly described by bed-partners and less frequently by patients unless injured.(3) The majority of REM sleep behavior disorder patients will eventually develop neurodegenerative conditions such as multisystem atrophy, dementia with Lewy bodies, and Parkinsonism.(4)
Epidemiology
The overall prevalence of idiopathic REM sleep behavior disorder (RBD) is estimated to be around 1% in the general population and 2% in aged individuals, with a median age at diagnosis between the 60s and 70s.(5)(6) It is predominantly observed in elderly males than females, with a male-to-female ratio of 9:1.(7) There is a strong association of RBD with multiple neurodegenerative disorders. As many as 76% to 81% of those who have RBD might later go on to develop more serious alpha-synuclein neuropathies, including multiple system atrophy, dementia with Lewy bodies, or Parkinsonism.(8) In a case series, about half of the patients with RBD converted to a neurologic disorder within 12 years.(9)
Pathophysiology
Sleep is a physiological state distinguished by decreased body movement, diminished attentiveness to the surroundings, and loss of consciousness.(10) Non-rapid eye movement (NREM) sleep and Rapid eye movement (REM) sleep are two distinct states that have distinct physiological, neurological, and metabolic characteristics. Rapid eye movements during sleep are the most noticeable feature of REM sleep, which is why it was given that name.(11)(12) In addition to rapid eye movement, REM is characterized by muscle atonia, which suppresses all muscle activity except for eye movement and middle ear muscle activation.(13)
The brain stem, specifically the pons and neighboring regions of the midbrain, is the primary structure for controlling REM sleep. Cholinergic neurons in the reticular formation’s pontine tegmentum are active during REM sleep. REM sleep is further regulated by orexin/hypocretin neurons and GABAergic neurons.(14) Special Electroencephalogram (EEG) features associated with REM sleep include low-amplitude desynchronized theta activity, sawtooth waves, pontine-geniculate-occipital (PGO) waves, and hippocampal theta activity. PGO waves happen either during REM sleep or when a person switches from NREM to REM sleep. The sleep state with the highest physiological arousal is known as REM sleep(13). Recent research has demonstrated that the regulation of dorsal medulla GABAergic neurons controls the REM phenomena in rodents.(15)
It is thought that nuclei in the medulla and lower pons, specifically in the perilocus coeruleus region, regulate the atonia. Through a flip-flop switch, this region causes REM atonia and directly innervates the spinal interneurons.(16) REM sleep atonia is induced by the hyperpolarization of spinal cord inhibitory interneurons, which are triggered by excitatory sublateral dorsal/subcoeruleus nucleus glutamatergic neurons. All of this results in inhibiting the spinal motoneuron pool. The REM-active and REM-inactive centers may be further stabilized by hypocretin neurons, which are thought to be found in the posterior hypothalamus.(17)
It is currently widely acknowledged that patients with REM sleep behavior disorder (RBD) lose the ability to control their Rapid eye movement (REM) sleep atonia. Nevertheless, it is still unclear what the fundamental mechanisms of RBD or REM sleep without atonia (RSWA) are. The spinal cord, brain stem, basal ganglia, cerebellum, and limbic/cerebral cortex all have afferent, integrative, and efferent systems that are involved in motor circuits. This sleep-related motor manifestation could be attributed to abnormal activation and disinhibition of these motor circuits. The red nucleus, pedunculopontine nucleus, and lateral dorsal tegmental nucleus can potentially be the origins of the physiological intermittent muscle twitches that occur during REM sleep. Clinical RBD may result from brain lesions in the dorsal pons, which lead to dysfunction of the sublateral dorsal/subcoeruleus nucleus, which has been shown to cause RSWA.(18)(19)
According to the Braak stage,(20) RSWA and RBD have been proposed to be caused by the buildup of Lewy bodies in the pontine and medullary structures, which occurs prior to the substantia nigra degenerates. Dementia and motor symptoms developed as Lewy bodies moved up to the cortex and substantia nigra.(21) On the other hand, RBD typically manifests years before cognitive dysfunction in Dementia with Lewy bodies (DLB), before Parkinsonism and other symptoms become apparent. It can be explained by the fact that the neocortex is involved in an earlier phase, and the nigrostriatal system is involved later or less in DLB compared to Parkinson’s disease (PD).(13)
The REM-active and REM-inactive centres and networks may be further stabilized by posterior hypothalamic hypocretin, and REM sleep behavior disorder (RBD) can also develop in the setting of hypocretin deficiency, as in narcolepsy type 1.(17) According to Braak’s hypothesis, which is based on the pathologic staging of Lewy body progression in Parkinson’s disease, the medulla and pons are where pathology arises, and this may be connected to the development of RBD and initial REM sleep without atonia (RSWA) in idiopathic RBD. The motor manifestation of Parkinson’s disease then changes as the disease spreads to the substantia nigra, and finally, as Lewy disease advances to the cortex, Parkinson’s disease (PD) dementia develops.(21)(22)
REM sleep atonia is controlled by pontomedullary structures such as the locus ceruleus (LC), laterodorsal tegmental nuclei (LDT), pedunculopontine nuclei (PPN), the magnocellular reticular formation (MCRF), and the sublateral dorsal nucleus (SLD).(23)(8) REM sleep without atonia (RSWA) can be developed from lesions in SLD, LC, and MCRF, and the complexity of motor behavior depends on the size and location of lesions.(18) In the case report of Erik K. St. Louis, given the lesion’s proximity to the sublateral dorsal nucleus (SLD) or its descending projections to the dorsal MCRF and spinal cord responsible for REM sleep atonia, as well as the strong temporal association of dream enactment behaviors (DEB) evolving shortly after other focal brainstem deficits, they assigned the patient’s RBD had been provoked by a discrete vasculitis lesion of the dorsomedial pons. This finding aligns with previously proposed criteria for determining lesional RBD causation.(24)
Clinical Presentation
Recurrent episodes of sleep-related vocalization and/or complicated motor actions during Rapid eye movement (REM) sleep are among the unique clinical symptoms of dream-enacting activity associated with REM sleep behavior disorder (RBD) that correlate with dreams.(25) RBD patients frequently behave violently or harmfully when they sleep, and they frequently act out their nightmares.(26)(27)(28) Thrashing, punching, kicking, and even falling out of bed are examples of the behavior, which also includes intentional, brief motions.(29) Additionally, the patients talk, yell, and make loud vocalizations. Normal dreaming causes a physiological loss of muscle tone in the skeletal muscles.(30) Loss of atonia in RBD leads to self-harm as well as harm to their bed partners. A thorough and in-depth history is necessary for the diagnosis of RBD.(31)
Patients with RBD have different dream content than the general population. They typically have more violent and obnoxious dreams. Until they or their bed companions are injured, patients tend not to think this is an issue.(32) While the rate of Sleep-related injuries occurred in 79% of patients in this series of patients.(4) Symptoms predominantly occur in the second half of the night when REM sleep is most prevalent and usually occur during the last REM sleep period.(4)
Risk factors
In both idiopathic and symptomatic categories, REM sleep behavior disorder (RBD) is strongly associated with neurodegenerative conditions, especially Synucleinopathies, including Parkinson’s disease (PD), Dementia with Lewy bodies (DLB), Multiple system atrophy (MSA), and pure autonomic failure.(33)(9)(6)(8)(34)(35)(36)(37) Compared to PD and controls, RBD patients tend to be obese and utilize antidepressants. This might arise because such individuals are more susceptible to presenting with sleep-disordered breathing disorders, particularly Obstructive sleep apnea (OSA), which could result in a concurrent earlier RBD diagnosis during sleep examinations.(37)
RBD is found to be associated with lower educational level, previous head trauma, occupational pesticide exposure, and farming are all risk factors for RBD, yet tobacco consumption, ischemic heart disease, and inhaled corticosteroids have all been associated with the disorder.(38)(39) The cross-sectional study of the association between REM behavior disorder and narcolepsy has suggested that 36% of people with narcolepsy have shown signs of RBD.(40) This relationship led to the detection of a substantial association between RBD and HLA class II genes.(41)(42)(43)
Diagnostic Criteria
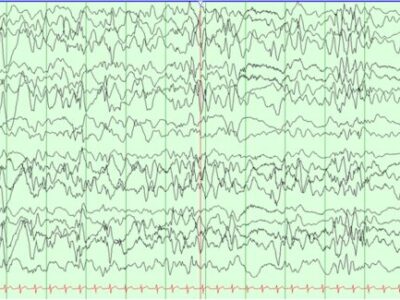
Although screening questionnaires or a history of abnormal sleep behaviors (particularly when obtained from the bedpartner) can be highly suggestive of REM sleep behavior disorder (RBD) in the appropriate context, formal diagnosis according to International Classification of Sleep Disorders-3 (ICSD-3) requires a Polysomnogram (PSG). This uses a combination of Electroencephalogram (EEG) and Electromyography (EMG) to identify the cardinal polysomnographic features of REM sleep without atonia (RSWA), in addition to abnormal behaviors during Rapid eye movement (REM) sleep, which are usually documented from a time-locked video recording during the PSG(44) where repeated episodes of complex motor behaviors or vocalization during REM sleep, an evidence of REM sleep without atonia (RSWA) on PSG. Although RSWA is not seen, the diagnosis of RBD can still be established as it strongly suggests additional clinical symptoms.(13)
The significance of REM sleep without atonia (RSWA) alone, without any overt behavioral component, is uncertain, although there is some evidence that it may be a precursor to REM sleep behavior disorder (RBD), representing, in a sense, a prodromal form of RBD itself that predicts future neurodegenerative conversion.(44) As the characteristic finding in RBD patients is increased muscle activity during REM sleep, an alternative diagnostic criteria for RBD diagnosis were proposed by the Sleep Innsbruck Barcelona (SINBAR) group. This guideline feature a cut point of any recorded and quantified Electromyography (EMG) activity in the muscles of the right and left flexor digitorum superficialis (upper limbs) and mentalis (chin) of 32% or greater during REM sleep was sufficient for the diagnosis of Idiopathic RBD and RBD associated with Parkinson’s disease (PD), using 3-s miniepochs.(45)
RBD can be secondary to several medications, most of which are antidepressants.(46)(47) When RBD is believed as a secondary cause of medication use, a diagnosis of RBD is still applicable.
Treatment
Management for REM sleep behavior disorder (RBD) includes several aspects to target, which are counseling for the patient and bedpartner if there, discontinuation of drugs causing RBD, safety measures in the bedroom, and pharmacological intervention as a final point.
To prevent harm or serious complications, all patients with RBD should receive counseling regarding bedroom safety measures. These measures include lowering the mattress to the floor or protecting against falls by placing mattresses or foam cushions on the floor, padding any sharp surfaces of bedside furniture, and keeping firearms out of the bedroom. Separate bedrooms may be advised in some situations to avoid bed partner harm. Some patients may benefit from a bed alarm system that could comfort and notify them during RBD activities, particularly if they also exhibit sleepwalking behaviors.(48) Discontinuation of drugs that may cause RBD when it is believed to be secondary, most of which are antidepressants.(49)
Melatonin receptor agonist “Melatonin” is the first-line treatment for RBD.(50) Melatonin stands out in symptomatic RBD treatment for patients with comorbid sleep apnea or problems with memory, and the recommended starting dose is 3 mg, which, with an average effective dose of 6 mg, should be gradually increased to the range of 6 to 12 mg at bedtime. It has been demonstrated that melatonin raises REM sleep atonia levels, diminishing REM sleep without atonia (RSWA).(51)(52)(53) Another effective treatment for RBD is to take 0.25 to 2.0 mg of clonazepam (Benzodiazepines) before bed. However, it does not seem to lessen RSWA; rather, it may alter complicated motor activities or dreaming. Clonazepam should be used cautiously by elderly people, especially those with Dementia with Lewy bodies (DLB), Parkinson’s disease (PD), and Multiple system atrophy (MSA), as it may exacerbate comorbid obstructive sleep apnea (OSA) and cognitive impairment.(54)(55)
Fortunately, melatonin has fewer side effects and is more tolerable than clonazepam; both have been demonstrated to prevent harm and lessen the frequency and intensity of RBD behaviors.(56)
The majority of side effects, such as headaches, daytime sleepiness, or sedation that lasts until the next morning, are dose-related and may frequently be avoided by reducing the dosage.(57)
REM sleep behavior disorder (RBD) and α-synucleinopathies
The most significant indicator of the prodromal stage of the neurodegenerative diseases is idiopathic REM sleep behavior disorder (iRBD), which is now thought to be the expression of the initial neurodegenerative process underlying synucleinopathies, such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA).(10)(58) According to large longitudinal cohort studies, 81–91% of patients with iRBD who are monitored for at least 14 years would either experience moderate cognitive impairment or a definitive neurodegenerative illness.(34)(59) iRBD is regarded as a neurodegenerative marker with limited sensitivity and high predictive value. According to estimates, the likelihood ratio of Polysomnogram (PSG)-proven iRBD for the onset of a synucleinopathy is 130, which is more than three times more than the likelihood ratio of striatal dopamine loss being detected by molecular imaging.(60) In contrast, only around half of all PD patients have gone through the prodromal stage of iRBD .(61)
Numerous clinical investigational studies have proven that patients with idiopathic RBD frequently exhibit symptoms or indicators that point to likely underlying synucleinopathy pathology. These symptoms include orthostatic hypotension, hyposmia, constipation, and abnormalities in gait, all of which have been linked to an increased risk of phenoconversion (Figure 1).(38) Furthermore, neuropsychological deficits in short-term memory, attention, dysexecutive, and visuoperceptual abilities seem to worsen over time.(62)(63) Neurophysiological indicators like electroencephalogram slowing and neuroimaging studies have also shown results that suggest underlying synucleinopathy pathology.(64)(65)
Functional imaging studies, including dopamine transporter uptake, have revealed decreased nigrostriatal putamenal dopaminergic uptake in patients with idiopathic RBD,(66)(67) as well as decreased cortical thickness and diffuse resting state metabolic network dysfunction with similar patterns to Parkinson’s disease (PD).(68)(69) Neuroimaging has also demonstrated altered neuromelanin signal intensity in the locus coeruleus/subcoeruleus nucleus region.(18)

Figure 1: Theoretical model of rapid eye movement sleep behavior disorder (RBD) and its relationship with different clinical manifestations of synucleinopathies.
In the future, idiopathic REM sleep behavior disorder (iRBD) is a condition that could benefit from disease-modifying treatments that are being developed with the goal of halting the degenerative processes that lead to the early development of synucleinopathies. The study of iRBD pathology can allow the understanding of biological alterations preceding the clinical manifestation of a synucleinopathy, accordingly foreseeing personalized treatments at a stage where cellular damage could be reversible.(70) In order to accomplish this, understanding which traits of iRBD are linked to pathological advancement and to accelerated or slowed phenoconversion is a must.(71)
The application of early neuroprotective measures and disease-modifying treatments is advantageous in iRBD, given that it represents a high-risk population for conversion to synucleinopathies. Numerous potential biomarkers, such as clinical, neurophysiological, genetic, CSF, serum, and neuroimaging, have been identified by recent longitudinal studies as useful for tracking the course of the disease and forecasting the conversion of iRBD into synucleinopathies (Figure.2). Nevertheless, it is still ambiguous how biomarkers function as iRBD predictors. The development of REM sleep behavior disorder (RBD) and its conversion into synucleinopathies may be anticipated with sensitivity and specificity using a combined multimodal biomarker model. To confirm these possible biomarkers and move closer to using them as endpoints in upcoming clinical trials, further investigation is needed, particularly large, multicenter, longitudinal studies.(72)

Figure 2: The main biological alterations associated with a greater likelihood of progression and/or iRBD patients’ phenoconversion to synucleinopathy.