Article Title: Intraventricular Brain Hemorrhage
Author: Yara Alswaiti
Editors: Yasmeen Alabdallat, Rawan AbuHammor
Scientific Editor :- Dr. Adam M. Abdallah
Keywords: IVH; Hemorrhage; CSF; Hydrocephalus.
Abstract
Intraventricular brain hemorrhage (IVH) refers to blood within the brain’s ventricular system (VS), a communicating network of cavities filled with cerebrospinal fluid (CSF) located within the brain parenchyma; this mainly includes the lateral, third, and fourth ventricles. IVH is associated with severe morbidity due to the risk of obstructive hydrocephalus.1 In this article, we aim to provide you with basic knowledge about how to assess and treat a patient with IVH.
Keywords: IVH; Hemorrhage; CSF; Hydrocephalus.
Introduction
Blood that refluxes into the ventricles from the subarachnoid space, the interval between the arachnoid membrane and the pia mater, is commonly unclotted and settles independent portions of the VS2. Bleeding restricted to the brain’s VS happens due to intracerebral hemorrhage rupture or subarachnoid hemorrhage that extends to the ventricles; this is most commonly a secondary symptom.
Primary intraventricular hemorrhage (PIVH) accounts for 0.31% of all stroke cases and 3.1% of all spontaneous intracranial hemorrhages. PIVH refers to nontraumatic intracranial bleeding in the VS and adjacent ependymal lining without a definite parenchymal component 3. PIVH is more commonly seen in preterm newborns than in adults. Secondary IVH refers to IVH with subarachnoid hemorrhage or other parenchymal hemorrhages 4,5. The underlying etiopathogenesis for adult PIVH is variable and often remains idiopathic. However, the common associations include hypertension, arteriovenous malformations (AVMs), aneurysms, moyamoya disease (MMD), coagulopathy, and arteriovenous fistula6. Due to the importance of this, the present review aims to provide you with basic knowledge about how to assess and treat a patient with IVH.
Epidemiology
Brain hemorrhage has the highest morbidity and mortality of any stroke subtype. Respectively, intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) account for about 15% and 5% of the 750,000 strokes occurring yearly in the United States, totaling more than 45,000 patients per year.7,8 About 45% of spontaneous ICHs and 25% of aneurysmal SAHs extend into the ventricles 9,10. For patients with both ICH and intraventricular hemorrhage (IVH), the expected mortality is 50% to 80% 11,12. In addition, patients with IVH are twice as likely to have poor outcomes (a modified Rankin scale [mRS] score of 4–6 at hospital discharge) and nearly three times more likely to die than the healthy controls 13.
Etiology
The quantity of blood inside the ventricles is closely associated with the severity of harm and the chance of survival. Furthermore, the quantity of bleeding into the VS is manifestly associated with the severity of the initial injury and the long-term implications of blood extending into the ventricles, keeping with the most current human clinical research 14.
A brain hemorrhage can be caused by aneurysms, arteriovenous malformations, small microaneurysms in the vessel wall, a coagulation profile, or simply increased blood pressure causing bleeding sites. As a result, a variety of disorders, such as trauma, tumors, and high blood pressure, can result in blood collections that restrict or occlude the intraventricular space 14.
Early intraventricular rupture is caused by bleeding at deep intracranial sites near the ventricles, but bleeding sites further distant from the ventricles may stockpile clotting blood before mechanical pressure, and hemorrhage cause a rupture into the ventricles. The actual rupture is typically linked with a clinically detectable loss of consciousness, frequently followed by death. The bigger the volume of blood clots injected into the ventricles in both canine and porcine IVH models, the greater the chance of animal death. Similarly, prolonged blood exposure to the ventricles causes altered consciousness as well as inflammation, fibrosis, and hydrocephalus at tissue level 14.
Although the etiology of IVH in neonates is unknown, it is believed to be caused by a shortage of oxygen to the brain, which may occur due to a traumatic birth or difficulties after delivery. Because the blood arteries in a premature baby’s brain are fragile and readily ruptured, bleeding might ensue. Also, IVH is very common in babies with respiratory issues or other preterm concerns 15.
Clinical presentation and prognosis
When clots distend the ventricular system, compress the nearby brain, or restrict cerebrospinal fluid (CSF) flow, hydrocephalus and high intracranial pressure result (ICP) 2. Common symptoms seen in IVH include apnea, bradycardia, pale or blue skin coloring, seizures, and anemia 15. Symptoms of ICH usually emerge suddenly, such as headache, weakness, disorientation, and paralysis, often on one side of the body. Blood builds up in the brain, putting pressure on it and interfering with its oxygen supply. This can quickly harm the brain and nerves. The significance of IVH as a clinical factor is linked to its negative outcomes such as coma, death, and long-term functional disability.
To clarify, blood in the VS contributes to morbidity in various ways. First, damage to the reticular activating system and thalamus during the acute phase of hemorrhage expansion causes a decreased level of consciousness. As a result, the coma appears to be prolonged with both a larger volume of blood in the ventricles and a more prolonged exposure 16,17. Second, ventricular blood clots blocking CSF conduits cause acute obstructive hydrocephalus, an immediately life-threatening condition limiting cerebral perfusion and potentially contributing to mass effect and resultant cerebral edema. Third, blood degradation products become embedded in the arachnoid granulations and may cause permanent occlusion and scarring, inhibiting CSF absorption and causing nonobstructive (communicating) hydrocephalus. Fourth, clotted blood may persist in the ventricles for several reasons related to coagulation and fibrinolytic pathways in the ventricular system 18.
After neonatal IVH, tissue plasminogen activator may be demonstrated, but fibrinolytic activity often is not seen for several weeks after IVH, and there appear to be insufficient concentrations of CSF plasminogen acutely 19. The fibrinolytic state of the CSF may impair the rapid, immediate evolution of blood clot resolution in the ventricles. In the study by Naff et al. of the kinetics of intraventricular clot resolution in 17 patients with IVH, the percentage rate of clot resolution over the first ten days was 10.8% per day 20.
The inflammatory reaction caused by blood breakdown products in the ventricles also may affect long-term cognitive function independent of clot volume or mass effect, as demonstrated in experimental IVH 21,22. In human IVH, significant cognitive deficits in SAH patients with IVH compared with those without IVH have been found on neuropsychological testing 23.
Diagnosis
Computerized tomography (CT) scanning is commonly used to diagnose intraventricular bleeding. Angiography, magnetic resonance imaging (MRI), or both are recommended if the underlying etiology of IVH is unknown, especially if thrombolytic therapy is being explored. Any patient with main IVH and no apparent etiology and any patient younger than 45 who has an IVH should have further imaging done. Also, any patient with IVH who has no evident reason based on history and CT scans should have vascular imaging done, especially if they are 45 years old or younger than 24.
Although IVH numerical values help compare IVH volumes among patients, ventricular distention, concomitant parenchymal damage, and the underlying cause of bleeding are more closely connected to clinical status and prognosis 24.
All neonates born before 30 weeks should have a routine head ultrasound. The test is performed once between the ages of seven and fourteen days. A second standard ultrasound is recommended when the baby is supposed to arrive. When a baby’s health abruptly deteriorates, especially in the first week of life, IVH should be explored. A head CT is advised if a term infant exhibits symptoms after a difficult birth, a low blood count, or other evidence of bleeding difficulties 25, as shown in Figure 1 and Figure 2.
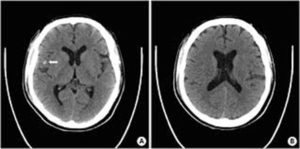
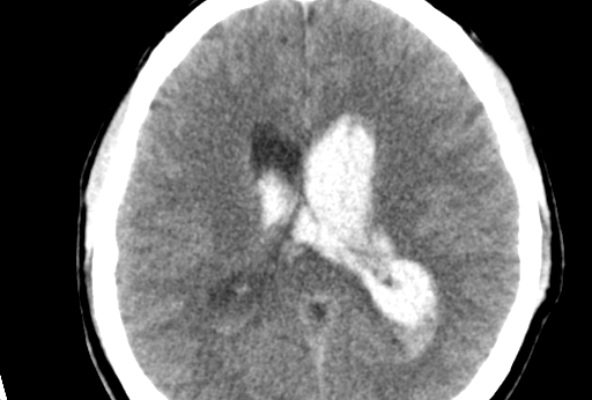
Figure 1: traumatic intraventricular hemorrhages in the frontal horn and body of bilateral lateral ventricles 40.
Figure 2:
A Case of Contrast Leakage Mimicking Intraventricular Hemorrhage in a Patient with Intravenous Thrombolysis 39.
IVH is a Severity Factor in ICH
Tuhrim et al. initially confirmed IVH as an independent risk factor for 30-day mortality after ICH and developed a model in which IVH volume contributed significantly to outcome prediction together with GCS score 26. Patients with IVH and ICH had a lower initial GCS score and larger ICH volume. Other contributors to the outcome included the number of ventricles containing blood and blood in the fourth ventricle. Subsequent prospective studies have continued to define the presence of IVH as an independent risk factor for mortality and poor functional outcome 27,28. In addition, the Tuhrim algorithm continues to perform better than any other outcome prediction algorithm when compared directly with independent data sets 29.
Recently, three large randomized controlled trials reconfirmed IVH as a severity factor. The Surgical Trial in ICH (STICH) enrolled 964 subjects to test the value of early surgery versus medical management. IVH occurred in 42% of STICH subjects (n=377); IVH with hydrocephalus occurred in 23% (n=208). Both were strongly associated with poor outcomes: 31% of patients without IVH and 15% with IVH had a good outcome (P<0.00001) 30. When IVH and hydrocephalus were combined, the percentage of patients with a good outcome fell to 11%. Similarly, in the phase 2b proof-of-concept study of recombinant activated factor VII (rFVIIa) for ICH (n=399), IVH occurred in 49% of subjects, in whom mRS scores were consistently worse; 80% of these subjects were vegetative or dead 31. In the multicenter Factor Seven for Acute Hemorrhagic Stroke (FAST) trial, the presence of IVH again was associated with increased mortality 32.
Hypertensive Brain Hemorrhage and IVH
In one-third to one-half of patients, spontaneous hypertensive ICHs are linked to secondary IVH. Chronic arterial hypertension is the most significant risk factor for this arteriopathy, which usually affects the lenticulostriate and thalamoperforating arterioles; other risk factors include moderate to heavy alcohol consumption and anticoagulant medication. Thalamic, putaminal, or caudate hemorrhage that spreads into the lateral or third ventricles is the most common complication of IVH. IVH has been linked to more significant parenchymal hematomas, midline shift of brain structures, and poor clinical outcomes in this situation 33.
IVH of the newborn
Intraventricular discharge of the infant is seeping into the liquid-filled regions, or ventricles, encompassed by the brain. IVH of the newborn is most commonly found in preterm babies, and the smaller and more premature the baby is, the greater the risk of IVH. This is because the blood arteries in premature infants’ brains have not yet fully matured and are extremely weak. IVH is uncommon at birth, and if it does occur, it usually happens within the first few days of life 34, as in Figure 3 which shows ultrasound images of the four grades of intraventricular hemorrhage in the newborn.

Figure 3: Germinal matrix hemorrhage and intraventricular hemorrhage (GMH-IVH) in the newborn
Germinal matrix hemorrhage and intraventricular hemorrhage (GMH-IVH) in the newborn
Intraventricular hemorrhage (IVH) of newborns is more common in premature babies who have had physical stress, such as respiratory distress syndrome, pneumothorax, and high blood pressure 25. IVH occurs in 45% of extremely premature neonates. 96% of IVH in neonates happens in the first four days. IVH is often described in four grades, depending on the amount of bleeding 34, as shown in Table 1.
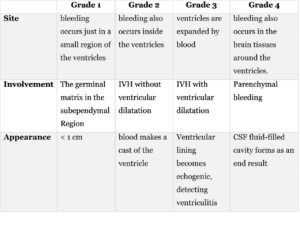
Table 1: describes IVH severity grades depending on the amount of bleeding.
Management and Treatment
In animals, removing the clot reduces inflammation, edema, and cell death, restoring cerebrospinal fluid flow and lowering intracranial pressure 14. Because systematic attempts to remove blood have not been done in extensive clinical studies on a broad scale, clinical knowledge about the benefits of intraventricular hemorrhage (IVH) removal in people is limited 14.
The CLEAR IVH trial treated intraventricular hemorrhage with a recombinant tissue plasminogen activator administered via a catheter. This catheter-based IVH treatment aligns with the theory that reducing intraventricular clot severity reduces disease severity by lowering mortality 35.
Lower rates of mortality have been routinely seen when a treatment strategy includes the use of an intraventricular catheter to keep ICP below normal limits and with the use of efforts to clear clots by administering low doses of thrombolytics 12.
External ventricular drainage (EVD) is the conventional treatment for substantial IVH (i.e., associated hydrocephalus). In patients with severe IVHs, intraventricular fibrinolysis or thrombolysis (IVT) with an enzyme such as rt-PA delivered through an external drainage catheter appears to speed IVH clearance, maintain catheter patency, and improve ICP management 36.
Following this were reports and case series of intraventricular fibrinolysis (IVF) in human patients, which included injections or infusions of urokinase or recombinant t-PA (rt-PA) through external ventricular drainage catheters for IVH caused by aneurysm and AVM rupture, chronic hypertension, ventricular catheter insertion, trauma, and germinal matrix hemorrhage in the newborn 21,37.
Permanent management methods currently used include VP shunt insertion, although it is associated with a high mortality rate among pediatrics. Endoscopic third ventriculostomy (ETV) is another technique used especially for patients with obstructive hydrocephalus, however, it is not considered highly successful38.
Conclusion
Despite the fact that the kind, dose, and duration of fibrinolytic treatment varied, these exploratory investigations found that IVF increased IVH clearance and appeared to be safe. In a case series of primarily aneurysm patients, rt-PA clearance of IVH was also found to help control increased ICP, particularly in younger patients with limited intracranial compliance or those in poor neurologic condition due to extensive hemorrhage 38. Organized, multicomponent intensive care has improved and become more widely available, including acute blood pressure control and support for impaired cardiorespiratory functions 14.