Title of article: Lacunar Stroke
Author: Ala’a Shakkah.
Editors: Almutazballlah Qablan, Sadeen Eid.
Reviwer: Philip Sweidan.
Keywords: Lacunar stroke, Lacunar infarct, Lacunar syndrome, cerebral small vessel disease.
Overview
Lacunar stroke or lacunar cerebral infarct (LACI) refers to a well-defined subcortical ischemic occlusion at the level of a single perforating artery generally of <20 mm. [1,2] It is the most common clinical entity occurring because of angiopathies that affect microcirculation. [2] Also, it’s an indicator of cerebral small vessel disease and makes up 25% of ischemic strokes. [3] Actually, one out of every four to five individuals with ischemic infarction had a diagnosis of lacunar stroke. [2] lacunar infarcts are small in size; they are most of the time asymptomatic. However, the location and accumulation of multiple lacunar infarctions play a role in causing significant physical and cognitive disabilities. [4]
The arteries that are usually affected by changes that result in lacunae include lenticulostriate arteries, anterior and median branches of the cerebral artery, thalamoperforating arteries, branches of the posterior cerebral artery, and paramedian perforating arteries, and branches of the basal artery. [1]
One of the five classic lacunar syndromes is typically present in patients with lacunar stroke, and these syndromes include pure motor hemiparesis, pure sensory syndrome, sensorimotor syndrome, dysarthria-clumsy hand, and ataxic hemiparesis. However, in some cases, patients may suffer from an atypical lacunar syndrome. [5]
Pathology and etiology
Small cerebral infarction leading to a lacune is generated by an alteration of the blood flow in the distribution area of the penetrating arteriole. [6]
1- Microatheroma: The most frequent mechanism of arterial stenosis causing symptomatic lacunes is an atheromatous alteration of the artery wall. [7] Basically, the affected region is the proximal part of larger, 200–400 m in diameter perforating arterioles. The Occlusion of these branches results in bigger lacunar infarcts. [8]
2- lipohyalinosis: there is a hypertrophy of smooth muscle and other connective tissue components, as well as a thickening of the media of small arteries with fibrinoid deposition.[4] Many of the smaller lacunes, especially those that are clinically asymptomatic, are caused by it because it affects the smaller perforating arteries, those that are less than 200m in diameter. [7,8]
3- Fibrinoid necrosis: it results from an abrupt and significant elevation in blood pressure (BP) and is detected in the brain’s arterioles and capillaries in some conditions such as hypertensive encephalopathy or eclampsia. [7,8]
4- Embolic obstruction: Despite being a rare cause, embolic blockage of perforating arteries or the vessel that arises from these arteries may be caused by one of two mechanisms:
- Embolism of arterial origin: carotid or aortic atheromata may cause microemboli of atheroma fragments of cholesterol crystals, resulting in lacunar infarcts. [9]
- Embolism of cardiac origin: Autopsy-based series have revealed that emboligenous cardiopathy, particularly atrial fibrillation, rheumatic valve disease, and non-bacterial thrombotic endocarditis, are unusual causes of lacunar infarction. [8,10]
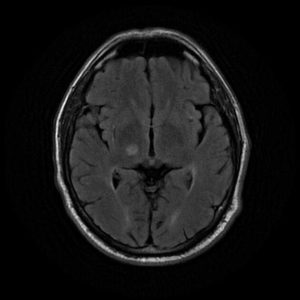
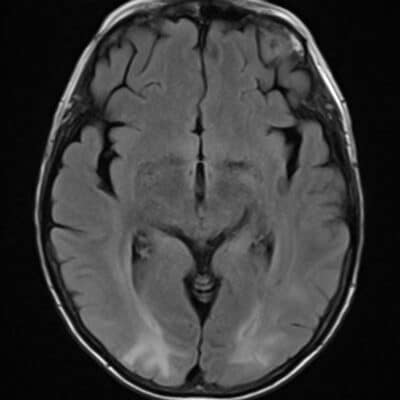
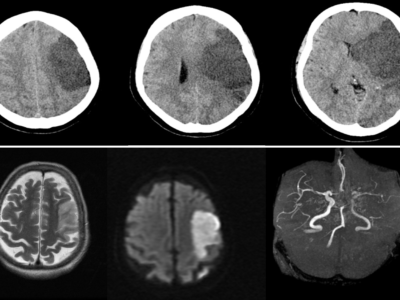
Figure 1: high signal intensity on the right thalamus (FLAIR) indicates lacunar cerebral infarcts. 11
Signs and Symptoms
Infarctions of the lacunar type in the brain can manifest in a variety of ways including asymptomatic lacunar infarcts, episodes of transient cerebral ischemia, and neurological deficits manifested as a lacunar clinical syndrome. [6]
1- Asymptomatic lacunar infarct
Patients with hypertension are more likely to experience asymptomatic lacunar infarctions, which are frequently numerous and brought on by lipohyalinosis. The most frequent clinical form is silent lacunes. [12] In fact, 42% of individuals with initial lacunar strokes have silent lacunar infarcts identified by MRI. [13]
2- Transient Ischemic Attack (TIA)
Lacunar infarcts can present as a TIA, which manifests as focal neurological impairments that last shorter than one hour. According to the standard definition of a TIA, which is “a cerebral malfunction of an ischemic nature lasting no longer than 24 h,” lacunar infarctions may make up 29–34% of all TIAs. [14-16]
3- Lacunar clinical syndrome
Pure motor hemiparesis, pure sensory syndrome, sensorimotor stroke, ataxic hemiparesis, and dysarthria-clumsy hand, in decreasing order in terms of occurrence, are clinical syndromes in patients with lacunar stroke. [6,17]
-
Pure motor hemiparesis
The most frequent lacunar form is pure motor hemiparesis, commonly referred to as a pure motor stroke. A pure motor stroke makes up 50% of all lacunar syndromes and 12.7% of all patients with their first-ever stroke in an acute stroke registry. [18] The most prevalent topographies are those of the posterior limb of the capsule, corona radiata, and pons. [18-22]
It is characterized as a total or incomplete paralysis of the face, arm, and leg on one side, unaccompanied by sensory symptoms, visual field impairment, dysphasia, or apractagnosia.[10]
-
Pure sensory syndrome :
Manifests with a sensory deficit (hypoesthesia) and/or an irritable sensory disturbance (paresthesia), which impairs either deep or surface sensations, or both. [6,23]
-
Sensorimotor syndrome:
The complete (faciobrachiocrural) or incomplete pyramidal syndrome, also known as a sensorimotor syndrome or sensorimotor stroke, is accompanied by a whole or partial sensory impairment on the same side of the body. [12,24]
-
Ataxic hemiparesis
It is the presence of a pyramidal syndrome concurrent with a homolateral ataxic syndrome. In some circumstances, a temporary sensory impairment may coexist with motor symptoms; this condition is known as ‘ataxic hemiparesis with hypoesthesia’. [25]
-
Dysarthria–clumsy hand
A rare lacunar condition with a good prognosis. [6,26] Clinical signs include homolateral hyperreflexia with Babinski’s sign, homolateral dysarthria with central facial weakness, and weakness of the hand with impairment of manual ability activities without a significant accompanying motor deficiency. [6,26,27]
Diagnosis
Anamnesis, neurological, neurovascular, and general physical examinations, as well as diagnostic techniques, are used to make the diagnosis of lacunar infarction. These findings allow for the diagnosis and causation of lacunar infarction, both of which are critical for treatment. [5]
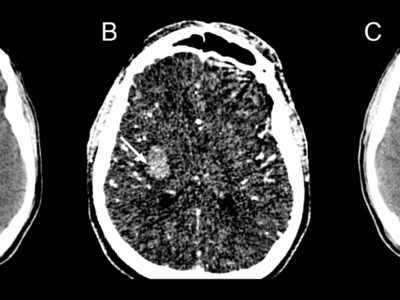
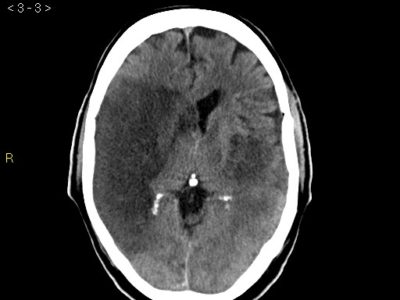
Due to its small size, lacunar ischemia is difficult to detect by CT scan within the first 24 hours. [4] If detected, lacunar strokes appear as poorly defined hypodensities on a CT scan unless the acute stroke also has a hemorrhagic component. [4] On a non-contrast head CT, a hyperdensity in a larger artery indicates the presence of a thrombus inside the arterial lumen. [4]
The initial brain CT is helpful in ruling out other conditions that share the same clinical symptoms and could be mistaken for a lacunar infarct, such as an extensive cerebral infarction, intracerebral hemorrhage, subdural hematoma, cerebral angioma, an expansive intracranial process, or demyelinating disease. About one out of every five cases of lacunar syndromes involve this. [6,28,29] For the detection of lacunar infarction in acute and subacute conditions, MRI is a better imaging technique. [30] The MRI diffusion-weighted image (DWI) has the highest degree of diagnostic precision during the acute phase. [30]
Differentiating between acute and chronic infarctions is much easier by MRI-DWI. [30] Lacunes show up as focal areas of reduced signal intensity on T1-weighted images and as focal areas of increased signal intensity on T2-weighted imaging in an acute context. [4] The assessment of underlying stroke risk factors is aided by a blood glucose levels test, electrocardiogram, complete metabolic panel, complete lipid panel, complete blood count with platelets, troponin, prothrombin time, and international normalized ratio (INR). [4]
Treatment
The fundamentals of treatment for an acute lacunar stroke are essentially similar to those for an acute ischemic stroke. [31,32] Assuring medical stability and determining the potential for thrombolysis are the initial objectives of acute-stage treatment. [31,32] If given to ischemic stroke patients within 4.5 hours of the onset of symptoms, tissue plasminogen activator (TPA) improves outcomes. [31,32] Intravenous thrombolysis is a significant step in the treatment after intracerebral bleeding has been ruled out. [31,32] TPA effectively treats an acute lacunar infarct. [31,32]
CTA or MRA can be helpful in identifying the potential for mechanical thrombectomy if more than 4.5 hours passed since the onset of symptoms began, there is suspicion of intracranial artery blockage in the anterior circulation, and the candidates are selected from those who had the onset of their symptoms between 6 to 24 hours before they were known to be well. [46]
Antiplatelet therapy is recommended for lacunar stroke, dual antiplatelet treatment (DAPT) with aspirin and clopidogrel might be started within 24 hours after the onset of symptoms and continued for 21 days in order to treat patients who present with an acute lacunar infarct outside of the tPA window and are consistent with a non-cardioembolic stroke. [4] For 90 days after the onset of symptoms, DAPT is useful in reducing recurrent ischemic stroke. [4] Antiplatelet therapy should be postponed for 24 hours in individuals who had tPA. [4]
The management of hypertension is necessary when the blood pressure is noticeably elevated (more than 220/120 mmHg). [4] Any outpatient on antihypertensive medications should also be held at admission for the first 24 hours unless other comorbid conditions demand the immediate lowering of blood pressure. [4] The optimal blood pressure for patients receiving tPA or undergone thrombectomy is below 185/110 mmHg. [4]
Blood glucose levels between 60 to 180 mg/dL are advised for blood sugar management in order to maintain euglycemia. [4] Both hyperglycemia and hypoglycemia need to be watched for and treated. [4] The preferred HbA1c target range is 6.5–7. [4]
Prevention
The use of hypertension drugs, diabetic control, cholesterol-lowering medications, quitting smoking, dietary changes, weight loss, and exercise are among the primary stroke preventive strategies. [33]
Antithrombotic medications such as aspirin, clopidogrel, extended-release dipyridamole, and ticlopidine are used in secondary prophylaxis with the management of underlying risk factors. [33] Patients with lacunar strokes who use antiplatelet medications experience lower recurrence rates. [33] Secondary prevention techniques include blood pressure control, diabetes treatment, cholesterol management, quitting smoking, weight loss, and regular exercise. [34] It is advantageous to lower blood pressure to a target of less than 130 mmHg for the systolic reading. [34]
Risk factors
1- Age: The majority of patients with lacunar infarction are between the ages of 55 and 75. [35] When the frequency of lacunar infarction was evaluated in four age subgroups (patients under 65, 65-74, 75-84, and 85 years old), lacunar infarction was the most common stroke subtype in those under the age of 65 years, and in the group aged 65–74 years. [35]
2- Sex: In most investigations, regardless of the patient’s age, the incidence of lacunar infarction is higher in males. [36-38]
3- Hypertension: The lipohyalinosis that leads to lacunar infarcts is both a result of hypertension and a risk factor for atherosclerosis. [12] Fisher identified hypertension as a specific etiology of lacunar infarcts, which was present in 97% of patients. [12]
4- Diabetes Miletus: Despite having a lower prevalence than hypertension, diabetes is a risk factor for and likely the cause of several lacunar infarctions: 11% in the Fisher series However, compared to the other stroke subtypes, lacunar infarcts had a higher rate of diabetes mellitus. [6,12]
5- Heart disease: Ischemic heart disease, which has an incidence of 26% in our series, [37] and ranges between 17% [38] and 39% [39] in other series published in the literature, is a cerebrovascular risk factor and a sign of widespread atherosclerosis. [5]
6- Carotid atherosclerosis disease: In 8-13% of lacunar infarcts, carotid atherosclerosis has been reported as carotid stenosis, with a lumen decrease (>50%). [40,41]
7-Transient ischemic attacks: 20% of patients with lacunar infarcts in clinical investigations had prior transient ischemic episodes (TIAs) reported. [6]
8-Smoking: increases the risk of developing lacunar infarction, with incidence rates ranging from 28% [38] to 68%. [39]
Prognosis
Previous research indicated that lacunar stroke has a better prognosis than other types of strokes. It has a high rate of survival, a low rate of recurrence, and a generally positive functional recovery. [42] The prognosis for lacunar infarcts is generally favorable. [42]
Recent research revealed that the prognosis of lacunar stroke seems benign in the early stages of the disease. [43,44] However, the long-term prognosis of lacunar stroke increases the probability of mortality, primarily from cardiovascular reasons. [43,44] Similar to other types of strokes, recurrent stroke carries a similar risk. [43,44] Recurrent small vascular disease patients are more likely to experience memory loss and dementia. [43,44]
Recent updates and Ongoing research
A clinical trial is now being conducted to determine the safety and efficacy of two of these medications, cilostazol and isosorbide mononitrate, in treating lacunar stroke. [45] Currently, cilostazol is prescribed to patients with peripheral arterial disease. [45] Additionally, isosorbide mononitrate is utilized to treat illnesses including angina. [45] 200 patients will participate in the trial and receive either cilostazol, isosorbide, or both treatments. [45] According to experts, these medications might reduce the damage that a stroke does to the arteries in the brain MRI scans will be performed on trial participants to determine how these medications affect the small blood arteries in the brain. [45]