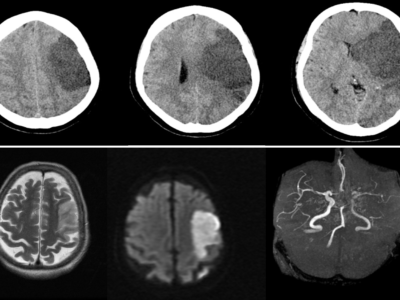
Article Topic: Intracerebral Hemorrhage (ICH)
Author name: Mohammad Ahmad Sawaftah
keywords: Cerebrovascular accident , hemorrhagic stroke , intracerebral hemorrhage
Overview and Epidemiology:
Stroke is an acute vascular injury of the brain tissue, causing impaired blood delivery either due to ischemia or hemorrhage (intracerebral hemorrhage ICH and subarachnoid hemorrhage)(1). It accounts major numbers of deaths annually and is also a leading cause of disability globally(2).
In 2017, more than 100 million cases of stroke were reported worldwide, around 80% of them were ischemic stroke, and the rest were classified as hemorrhagic stroke (17.9 million cases of intracerebral hemorrhage and 9.3 million cases of subarachnoid hemorrhage). Although intracerebral hemorrhage doesn’t have the highest prevalence rate, it does have the highest mortality rate compared to the other types (around 50% of stroke deaths)(3).
Intracerebral hemorrhage is defined as bleeding inside the brains parenchymal tissue, its spectrum of presentation is wide and varies depending on the blood vessel supplying the site of the lesion. It could be motor or somatosensory deficit, loss of consciousness, cognitive dysfunction, headache, psychological changes, etc. The presentation doesn’t differentiate the type being ischemia or bleeding, that’s why non-contrast CT is essential, to determine the etiology and subsequently the management (4).
Pathogenesis and Etiologies:
- Trauma: Direct blood vessels injury that could be anywhere in the brain.
- Hypertension : The main cause of ICH in adults affecting the small vessels is due to long standing hypertension, causing fibrinoid necrosis and lipohyalinosis of the vessel walls, making it weak and leaky(4).
- Amyloid angiopathy : is the main cause of spontaneous ICH in elderly, affecting small vessels due to amyloid deposition with aging, making the blood vessels weaker and fragile(5).
- Arteriovenous malformations : the main cause of spontaneous ICH in children(6).
- Iatrogenic : reperfusion therapy after ischemic stroke can be complicated by reperfusion injury, one of these injuries could be hemorrhage(7)(8).
- CNS tumors: ICH is a common consequence of brain tumors, could be due to tumor cells invading the cerebral blood vessels, or vascular endothelial growth from the tumor itself. More common in metastatic brain lesions (Thyroid papillary carcinoma, Hepatocellular carcinoma), but primary brain tumors can cause it as well ( pituitary adenoma, polycystic astrocytoma, glioblastoma)(9)(10).
- Vasculitis : an inflammation of cerebral vessels, can be Primary angiitis of CNS (without involvement of systemic vasculitis) or secondary vasculitis (with vasculitis affecting other organs like SLE, RA, Bechet disease)(11).
Risk factors:
- Hypertension(4).
- Smoking(12).
- Low LDL and cholesterol : although hyperlipidemia is a risk factor for ischemic stroke, low LDL and cholesterol are considered risk factors for ICH( 13).
- Bleeding tendency : as a result of using anticoagulation drugs, especially warfarin and Tissue plasminogen activator (tPA) which have a higher risk of causing ICH than direct oral anticoagulants(14). Coagulopathies as well (inherited coagulopathies, neoplasia related coagulopathy, acute leukemia, etc.)(15).
- Substances and drugs: like cocaine or amphetamines(16).
- Age: due to higher risk of having hypertension (4) and amyloid angiopathy (5).
- Male gender(12).
- High alcohol consumption(12).
- Chronic kidney disease : low glomerular filtration rate of the kidney is associated with increased intracerebral hemorrhage(17).
- Genetic factors :although the genetic factor is considered a weak influencer on ICH cases, but APOE (seen in many ethnicities) and PMF1 ( more in Europe) genes are counted ones(18).
Clinical presentations and complications:
Cerebrovascular accident presentations are somehow classical, and it could not be determined whether it’s the ischemic or hemorrhagic type, it could be widely scattered according to the affected blood vessel and the area that’s supplied by that vessel, on the other hand it could consist of multiple symptoms and signs depending on how much the hematoma has spread. The presentation could consist of: headache, loss of consciousness, altered mental status, hypersomnia, cognitive disturbances, psychological problems (4)focal and neurological deficit; cranial nerves motor or sensory function loss, motor or somatosensory defects.
Intracerebral hemorrhage is divided according to the location of the hemorrhage into lobar (cortical and subcortical areas) and non-lobar (deep brain structures; like thalamus, Pons or cerebellum)(19):
- Lobar ICH is caused due to cerebral amyloid angiopathy, AV malformations, tumors and less commonly hypertension, commonly seen in elderly people.
Presentation is according to the affected cortical lobe. Visual problems if occipital lesions are the ones affected, sensory loss due parietal lobe involvement, hearing and language dysfunction if temporal lobe, and decrease in memory function along with emotional and personality disturbance in frontal bleeding.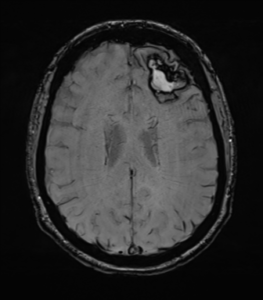
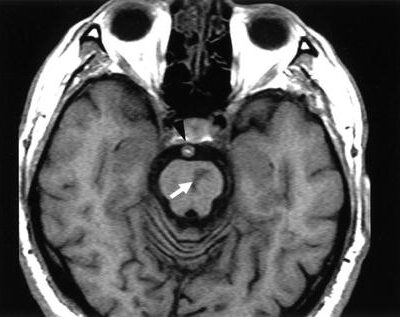
figure1: T1 weighted MRI showing a lobar Intracerebral hemorrhage ICH(20)
- Non-lobar ICH is also referred to as Hypertensive intracerebral hemorrhage. Mainly affects the internal capsule and basal ganglia, but also seen at the cerebellum and brain stem.
Presentation is according to the area of the brain affected, internal capsule lesions will cause motor or sensory defect or both, motor voluntary control and movement impairment is seen in basal ganglia hemorrhage cases, vertigo and ataxia are linked to cerebellar bleeding, hypersomnia and motor or sensory symptoms are seen in thalamic hemorrhages.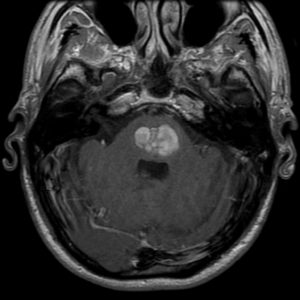
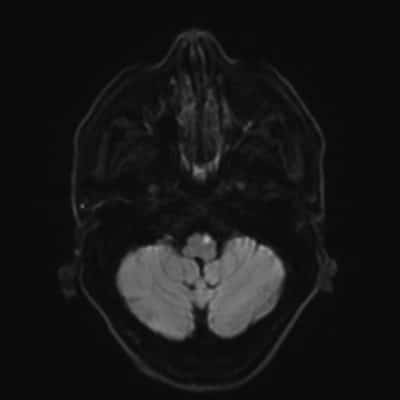
figure2: T1 weighted MRI showing pontine hemorrhage, which is considered as deep or non-lobar Intracerebral hemorrhage ICH(21)
Along with the clinical presentation of the ICH, possible complications can also happen and participate with the clinical picture overall:
- Brain edema and elevated intracranial pressure : either during the first day around the bleeding area and is associated with the hematoma expansion, or after several weeks(22).
high ICP happens due to brain edema, large hematoma, or hydrocephalus, presents as headache, dilated pupils and confusion, and sometimes Cushing’s triad can also be seen (hypertension, bradycardia, and irregular respirations)(23).
figure3: acute cerebellar hemorrhage surrounded by vasogenic edema(24).
- Cerebral ventricles compression and hydrocephalus and IVH: presents with vomiting, headache and visual impairment.
Happens due to hemorrhage compressing the CSF pathway or intraventricular hemorrhage, and may require shunting of the CSF in some cases(23). - Hematoma expansion: presents as deterioration of the case presentation and new neurological impairment, commonly seen during the first day, especially those on anticoagulants (23).
- Seizures :more frequently seen in cortical and subcortical hemorrhages, could be early due to irritations of the cerebral cortex, or later on due to scaring of the cortex(25).
- Infection : respiratory and UTI are commonly seen post ICH, respiratory infection is very important and highly correlated with the mortality rate, happens due to coma, intubation, and dysphagia(26).
- Deep venous thrombosis : ICH is frequently complicated with DVT especially in Asian ethnic group and females(27).
Workup and diagnosis:
Although stroke diagnosis is mainly clinical, distinguishing between the types of stroke is hard using the clinical presentation alone. Hence, further investigations are important as a work up for ICH patients:
- Laboratory tests: platelets count to find if there is a low platelets level.(15) PT and PTT : searching for coagulopathy.(15)
- Drug screening : should be suspected in young adults.(16)
- Serum glucose : hypo or hyperglycemia should be corrected.(41)
- EEG : to detect any epileptiform waves(25).
Imaging modalities(28):
Non-contrast CT scan:
Non-contrast CT is found to be very practical and very sensitive, making it the imaging modality of choice in acute stroke that can differentiate ischemic from hemorrhagic strokes. This is a very crucial step in these cases because the management is going to be different from ischemic stroke (thrombolytics are contraindicated is this case).
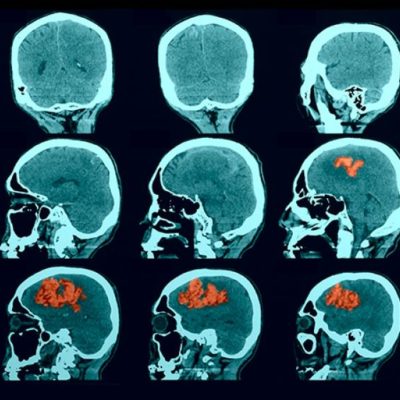
Hyperdense lesions on CT represent the hemorrhagic sites, in very acute stages of the case, and what makes it more practical is that it also detects the extension of the bleeding volume along with the site of the lesion, and possible complications can be revealed as well.
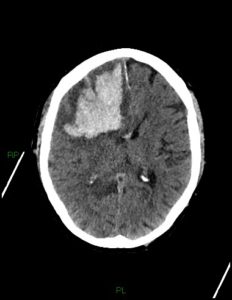
figure 4 : frontal lobe Intracerebral hemorrhage caused by coagulopathy(29).
Volume calculations:
ABC/2 method is a commonly used method to estimate the ICH volume, and was found to be very predictable to the outcome of the patient:
1- A is the longest diameter of the lesion.
2- B is the perpendicular diameter of the hemorrhage to A.
3-C is the number of the CT slices that exhibit the hemorrhage multiplied by the number of slices.
If the estimated volume was more than 30mL, in that case there’s a high possibility for a poor outcome. Although ABC/2 method is practical and useful in real practice, it still has its own falls. It assumes that the hemorrhage is ellipsoid with smooth dimensions, and not all the hemorrhages has regular or smooth edges, in fact almost all the hemorrhages will have irregularities, especially ICH due to oral coagulation drugs or coagulopathies, that puts the method in overestimation error.
Some would use Alternatives to ABC/2 formula such as 2.5ABC/6 in order to decrease the overestimation error and improve the accuracy (30).
CT scan and lesion aging and complications:
Regarding the aging of the hemorrhage, CT is also helpful during this part of the assessment using Hounsfield units. For example, during the first 2 days of the hemorrhage the CT will reveal a hyperdense lesion, and sometimes in large hematomas especially coagulopathy related or post-thrombolytic therapy ones it can show blood-fluid levels, which are horizontal lines easily differentiated and separate hypodense bloody serum layered above hyperdense settled blood, that could be seen on CT scan or MRI early on. One of the early markers that could be detected in the early 72 hours is the peripheral edema that appears as a hypodense area surrounding the lesion itself. Those early changes are frequently detected before the lesions density decreases with time and lesions size shrink down.
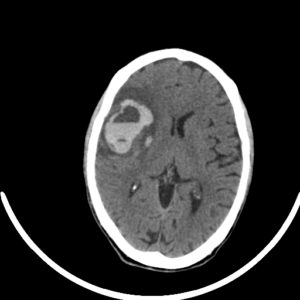
figure5: an early onset of ICH showing a blood-fluid level is evident that may be due to coagulation disorder or anticoagulation, with peripheral edema surrounding the hemorrhage(31).

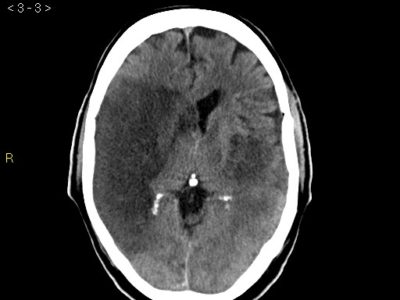
figure6: intraventricular hemorrhage due to ICH extension(32)
CT scan can also be helpful in identifying some complications after the ICH, like IVH and hydrocephalus
secondary to ICH extension, and are associated with bad outcomes of cases.
CT angiography (CTA) :
CTA is usually done in cases when vascular pathology is suspected, such as AV malformation or tumor that might cause the hemorrhage especially in lobar ICH, young age or absence of significantly known risk factors.
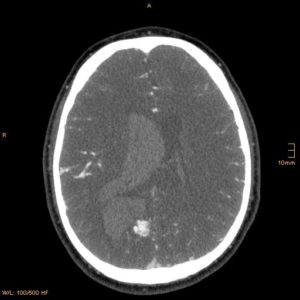
figure7 B: AV malformation that caused ICH in a young patient revealed by CTA axial section (33)

figure7 A: AV malformation that caused ICH in a young patient revealed by CTA sagittal section(33)
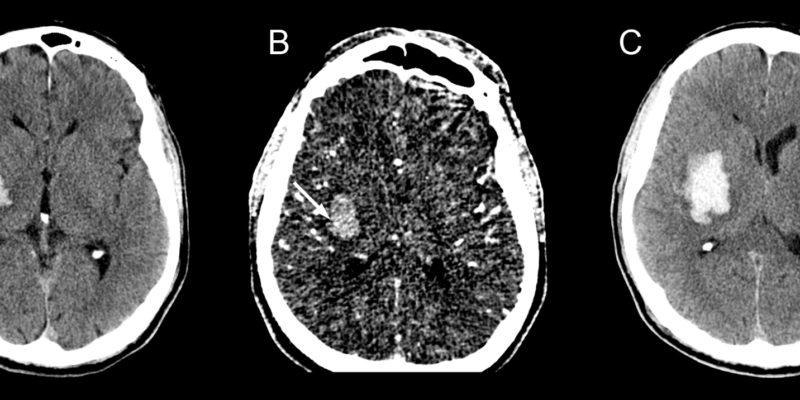
Spot sign:
Showing contrast enhancement at the lesion itself is called a spot sign. It has a very predictive outcome to the case, because the risk of hematoma expansion occurring increases when it appears, especially during the first 3 hours of symptom onset, appearance of spot sign increases the mortality rate as well.
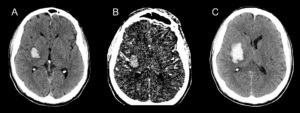
figure8: A: non contrast CT showing hemorrhagic lesion; B: CTA showing the spot sign, which is a hyperdense spot inside the hemorrhagic lesion; C: hemorrhagic expansion after the spot sign has appeared (34).
MRI Brain:
Although NCCT is more practical, and sometimes there are certain difficulties resulting in MRI not being an option, such as pacemaker placement and so on. MRI is still a sensitive tool for diagnosis of ICH, even 20 minutes after the onset of symptoms. In fact, susceptibility-weighted or gradient echo MRI are considered more sensitive than NCCT in subacute cases, and is the best modality to detect the actual aging of the ICH lesion, due to high sensitivity of molecular changes of hemoglobin in the lesion.
Aging of the lesion using MRI(35):
MRI is sensitive to hemoglobin changes inside the brains parenchyma, the composition of the lesion will predominantly form, either intracellular oxyhemoglobin (during the first 24 hours), intracellular deoxyhemoglobin (from days 1-3), intracellular methemoglobin (after day 3 till day 7), extracellular methemoglobin (from days 7-28), or hemosiderin (>28 days).
Those molecular changes are important because they will cause the radiological changes on the MRI at T1 or T2 sequence as the following:
- Hyperacute stage (before 24 hours): lesion will appear as isointense lesion at T1 , isointense-hyperintense at T2.
- Acute stage (from days 1-3): lesion will stay isointense at T1, however at T2 it will appear hypointense.
- Early subacute stage (from days 3-7): lesion becomes hyperintense at T1, although it will stay hypointense at T2.
- Late subacute stage (from days 7-28): lesion is hyperintense at both T1 and T2.
- Chronic stage (after day 28): the intensity of both MRI sequences will drop over time.
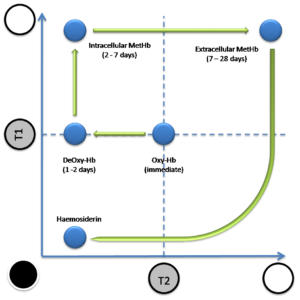
figure9 : this graph shows the hemoglobin color changes in T1 and T2 over time.
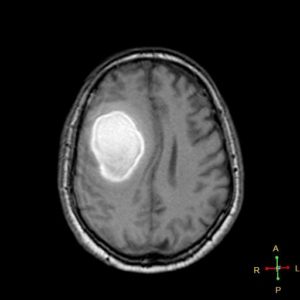
figure 10 A: subacute hypertensive ICH in T1 sequence(36)

figure 10 B: subacute hypertensive ICH in T2 sequence(37)
Figures 10A and f10B are showing the same lesion for the same patient at the same time, during this subacut phase where methemoglobin is extracellular (between day 7 and day 14), both sequences are hyperintense
MRI also has a high capability to detect the underlying causes of the hemorrhages such as AV malformations or tumors, making it a very useful imaging option in lobar ICH, young patients with no clear risk factors directed towards a certain etiology and micro bleeds that are related to vascular pathologies like hypertensive ICH and CAA that could not be detected easily by CT.
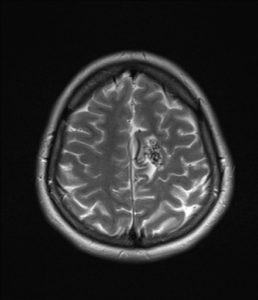
figure 11: This is a T2 weighted MRI showing AV malformation at the current section for a patient that had a hemorrhagic stroke(38).
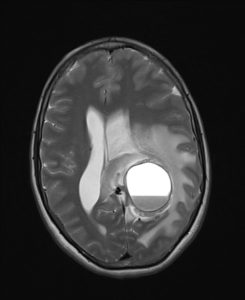
figure 12: Cystic glioblastoma with cerebral hemorrhage(39).
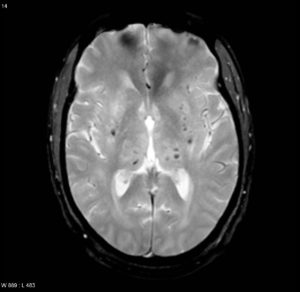
figure 13: Microbleeds due to chronic hypertension, which are silent small cerebral hemorrhages, a sign for vascular pathology due to hypertension or CAA(40).
Treatment and Management:
- Cardiopulmonary support: management initially should be focused on Airway, breathing, circulation and neurological deficit. Cardiopulmonary support is important, and screening for possible MI is required since in such cases patients may have concurrent MI, shock, etc.(41)(42).
- Intubation should be taken in consideration to secure the airways in patients with a high risk of aspiration, or when Po2 < 60 mmHg or Pco2 > 50 mmHg(41)(42).
- Neurological assessment and Glasgow coma scale as a baseline for follow up and to get an idea about the severity and prognosis (42).
- Blood labs should be sent: (CBC, KFT, coagulation profile, electrolytes and serum glucose (42).
- Urgent Imaging to differentiate ischemic from hemorrhagic strokes by CT or MRI (41).
- Blood pressure control: intensive blood pressure lowering is considered a safe approach when systolic blood pressure >220 mmHg, or when mean arterial pressure >150 mmHg.It also has a better outcome in cases where SBP ranges between 150-220. Acute hypertension treatment should then be considered to drop the SBP to 140 mmHg, but contraindications of treatment should be considered, kidney and neurological follow up must be obtained as well.
Short acting drugs are preferred to prevent overshoot hypotension, like Labetalol and nicardipine. Vasodilators such as hydralazine and Nitroprusside must be avoided due to their side effect of increasing the ICP (41)(42). - Dysphagia screening: dysphagia screening must be done to all patients before starting oral intake; this will prevent aspiration or pneumonia (41).
- Correction the coagulopathy: FFP, platelet transfusion, vitamin K administration depending on the coagulation problem present (15)(41)(42).
- Thrombocytopenia: platelet transfusion is indicated in severe low platelet count situation, when the count is less than 50000-100000µ
- ICH in patients taking IV heparin or LMWH: IV protamine sulfate is used as a reversal of the coagulopathy, with close vital signs monitoring because it can cause hypotension.
- ICH due to warfarin: warfarin causes increased INR value and correcting this elevation is the aim to decrease the hemorrhage tendency. Achieved by stopping warfarin, administering IV vitamin K infusion, using either FFP or PCCs, since vit K alone needs 2 hours to give a reversal action. PCC is generally preferred over FFP and considered more practical since it doesn’t require cross-matching and large volumes to correct the INR to the target value( <1.5), has faster correction ability of INR than FFP, and has less complications than FFP. After giving drugs INR should be monitored for follow up.
- ICH due to new anticoagulants: direct thrombin inhibitor (dabigatran) or factor Xa inhibitors (including rivaroxaban, apixaban, and edoxaban).
Idarucizumab, a humanized monoclonal antibody fragment against dabigatran (direct thrombin inhibitor) is approved by FDA for emergency situations.
Andexanet Alfa (Andexxa) is an antidote for patients treated with rivaroxaban, apixaban and edoxaban.
- Fever: fever is commonly seen in ICH patients, especially those with IVH. It’s related to the higher possibilities of hematoma spreadingcausing worse outcomes (41).
- ICH and DVT prophylaxis: Intermittent pneumatic compression has a better outcomein preventing DVT in comparison with the graduated compression stocking which didn’t give beneficial results in DVT prevention. That’s why intermittent pneumatic compression is recommended in all ICH cases in the first days of admission (41).
- Treatment for edema and high ICP is done bya using a dehydrant agent like mannitol, hyperventilation and keeping the head elevated at 30º to optimize cerebral perfusion and ICP. Steroids should not be used (22).
- Correction of the blood glucose level: hyperglycemia in ICH is associated with bad results, and decreasing the glucose levels in hyperglycemia decreases the hematoma spreading risk. As a general rule for glucose monitoring, treat low or high blood glucose(41).
- Seizures: is possible event after hemorrhagic stroke, either clinical convulsions or epileptiform EEG changes in unconscious patients, that’s why EEG is recommended in cases with altered mental status or unconsciousness.
Many antiseizure drugs can be used in these cases, although phenytoin and valproic acid should be avoided due to worsening of neurological outcome and increase in the mortality rate. New antiseizure drugs have safer profile and less drug interactions like Levetiracetam and Lacosamide on the other hand. Overall, Post-ICH prophylactic antiepileptic drugs are not recommended (25)(41). - ICH patients should be admitted to the neurological ICU, or dedicated stroke unit, for closer follow up and monitoring of the case, and this has shown better functional outcome, and less mortality rate than the regular ward admissions (42).
- Rehabilitation should be considered for all patients who suffer from hemorrhagic stroke to help improve the disabilities and recover from them(since ICH is a leading cause of these disabilities). It will furthermore help the patients have a better functional life even without being fully recovered from the disability(41).
- Atrial fibrillation and ICH(43)Atrial fibrillation is normally associated with a higher risk of ischemic stroke. Thus, coagulation therapy is considered in AF patients. On the other hand, anticoagulation is associated with hemorrhagic stroke. Hence, balancing the risk of having ischemic and hemorrhagic strokes made us develop a new way to manage those patients with AF and a history of ICH. Has-bled score is an important method to evaluate this case.Has-bled score:
Has-bled score is a score developed to evaluate the risk of major bleeds that the atrial fibrillation patient could encounter in one year due to anticoagulation drugs. The score helps cluster the patients into low and high risk patients, and take a better discussion toward anticoagulation medications.
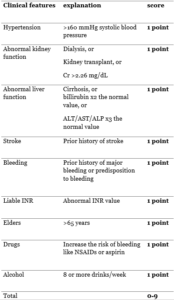
Has-bled score 3 or more indicates a high risk patient of bleeding, anticoagulants should be used with caution but not necessarily stop the drugs. Instead, clinicians can work more on the modifiable risk factors. Score of 5 or more is associated with a risk of bleeding of >12.5% during the year.
Prognostic Factors:
Hemorrhagic stroke is considered a serious disease with bad morbidity and mortality rates; it also causes a nonfunctional life to many people worldwide. There are some scoring systems for prognosis predictions, such as ICH score and FUNC score that are frequently used (42):
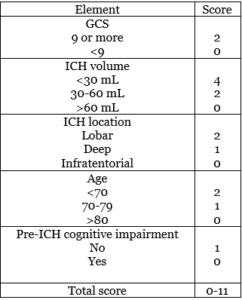
- ICH score: it’s used for 30-days mortality rate for ICH patients. Correlating the age of the patient, the hemorrhage volume and location, the presence of IVH, and the initial GCS with the prognosis (42).
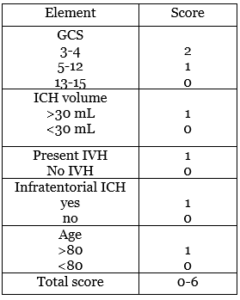
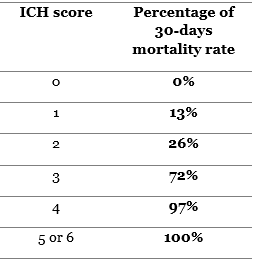
The higher the ICH score the higher the mortality rate will be. With a>70% mortality rate for ICH score of 3, and almost a 100% ICH score of 4 or more.
- FUNC score: used for 90-days functional independence. Very similar to ICH score components, but it includes pre-ICH cognitive status into consideration (42).
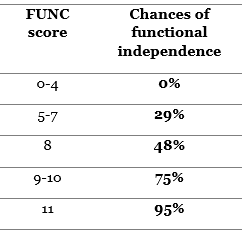
The higher the FUNC score the higher the chances are for the patient to have good functional outcomes. In fact, 4 or less FUNC score have 0% chance to achieve functional independence. Those with >8 FUNC score on the other hand have more than 75% chance of functional independence.
Generally, ICHs prognosis might worsen and have a higher complication and mortality rate due to the following situations:
- ICH due to coagulopathy especially warfarin. Which has a worse prognosis due to the larger hematoma occurring in these cases (15)
- Pneumonia post -ICH is associated with poor prognosis(26).
- Hydrocephalus complication after ICH is correlated with bad outcomes(44).
- Large sized hematoma (45).
- Old age (45).
- Initial low GCS (45).
- Hemorrhage volume more than 30 cc (45).
- Hyperglycemia with ICH(46).
- Spot sign: it has a poor prognostic factor, gives higher risk of possible hematoma spread (28).
Prevention(41)(47):
- Blood pressure control: controlling hypertension is considered the main action for prevention. It decreases the risk of having hemorrhagic stroke, and recurrence of the event later on.
- Anticoagulation: Aspirin isn’t recommended for primary prevention of ischemic stroke. Because it increases the risk of having cerebral hemorrhage, but continuing antiplatelet drugs in high risk ischemic stroke patients that had recent hemorrhagic stroke didn’t exhibit significant change in the risk of recurrence of the hemorrhage. Direct oral anticoagulants are superior in atrial fibrillation cases for prevention compared to warfarin, and have lower risk of causing ICH. The recommended timing to resume anticoagulation therapy in anticoagulants induced ICH is still unknown. Physicians must carefully balance the risks of recurrent ICH and thromboembolism in individual patients.
- Steering away from smoking, alcohol drinking >2 drinks/dayand illicit drugs are good lifestyle risk factor changes. Treating obstructive sleep apnea is also beneficial.
- Blood glucose control: glucose level correction is recommended. Treating hyperglycemia prevents bad outcomes as well.
Recent researches:
- Although Covid-19 patients are at a risk of thrombotic events during the course of the disease. Anticoagulation drugs (either as therapeutic or prophylactic doses),puts the patients at risk of having ICH with cerebral herniation or cortical ICH(48).
- Stroke patients are at a high risk of having disabilities that sometimes become permanent.However, DVT is more common in ICH and SAH than in ischemic stroke(49).
- In a non-randomized study testing platelet transfusion in patients under antiplatelet therapy and seeing the effect on hematoma expansion, the results exhibited no significant risk of hematoma expansion(50).
- Relapsing polychondritis is a rare disease in rheumatology. Presents usually as hearing loss due to auricular chondritis, but also can cause aortic aneurysm. extremely rare but reported cerebral vasculitis due to the disease, causing hemorrhagic stroke(51).
References:
- NHLBI,stroke. (n.d.). https://www.nhlbi.nih.gov/health-topics/stroke
- Katan, M., & Luft, A. (2018). Global Burden of Stroke. Seminars in Neurology, 38(2), 208–211. https://doi.org/10.1055/s-0038-1649503
- Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., Chamberlain, A. M., Chang, A. R., Cheng, S., Delling, F. N., Djousse, L., Elkind, M. S. V, Ferguson, J. F., Fornage, M., Khan, S. S., Kissela, B. M., Knutson, K. L., Kwan, T. W., Lackland, D. T., … Tsao, C. W. (2020). Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation, 141(9), e139–e596. https://doi.org/10.1161/CIR.0000000000000757
- Unnithan, A. K. A., & Mehta, P. (2020). Hemorrhagic Stroke.
- Charidimou, A., Boulouis, G., Gurol, M. E., Ayata, C., Bacskai, B. J., Frosch, M. P., Viswanathan, A., & Greenberg, S. M. (2017). Emerging concepts in sporadic cerebral amyloid angiopathy. Brain : A Journal of Neurology, 140(7), 1829–1850. https://doi.org/10.1093/brain/awx047
- Hutchinson, M. L., & Beslow, L. A. (2019). Hemorrhagic Transformation of Arterial Ischemic and Venous Stroke in Children. Pediatric Neurology, 95, 26–33. https://doi.org/10.1016/j.pediatrneurol.2019.01.023
- Piccardi, B., Arba, F., Nesi, M., Palumbo, V., Nencini, P., Giusti, B., Sereni, A., Gadda, D., Moretti, M., Fainardi, E., Mangiafico, S., Pracucci, G., Nannoni, S., Galmozzi, F., Fanelli, A., Pezzati, P., Vanni, S., Grifoni, S., Sarti, C., … Inzitari, D. (2018). Reperfusion Injury after ischemic Stroke Study (RISKS): single-centre (Florence, Italy), prospective observational protocol study. BMJ Open, 8(5), e021183. https://doi.org/10.1136/bmjopen-2017-021183
- Mokin, M., Kan, P., Kass-Hout, T., Abla, A. A., Dumont, T. M., Snyder, K. V, Hopkins, L. N., Siddiqui, A. H., & Levy, E. I. (2012). Intracerebral hemorrhage secondary to intravenous and endovascular intraarterial revascularization therapies in acute ischemic stroke: an update on risk factors, predictors, and management. Neurosurgical Focus, 32(4), E2. https://doi.org/10.3171/2012.1.FOCUS11352
- Fujita, K., & Matsumoto, S. (1980). [Intracerebral hemorrhage in brain tumors (author’s transl)]. No shinkei geka. Neurological surgery, 8(10), 929–934.
- Lieu, A. S., Hwang, S. L., Howng, S. L., & Chai, C. Y. (1999). Brain tumors with hemorrhage. Journal of the Formosan Medical Association = Taiwan Yi Zhi, 98(5), 365–367.
- Vasculitis foundation, Central nervous system vasculitis. (n.d.). https://www.vasculitisfoundation.org/education/forms/central-nervous-system/
- An, S. J., Kim, T. J., & Yoon, B.-W. (2017). Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. Journal of Stroke, 19(1), 3–10. https://doi.org/10.5853/jos.2016.00864
- Mustanoja, S., Strbian, D., Putaala, J., Meretoja, A., Curtze, S., Haapaniemi, E., Sairanen, T., Hietikko, R., Sirén, J., Kaste, M., & Tatlisumak, T. (2013). Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke, 44(8), 2330–2332. https://doi.org/10.1161/STROKEAHA.113.001829
- Emiru, T., Bershad, E. M., Zantek, N. D., Datta, Y. H., Rao, G. H. R., Hartley, E. W., & Divani, A. A. (2013). Intracerebral hemorrhage: a review of coagulation function. Clinical and Applied Thrombosis/Hemostasis : Official Journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis, 19(6), 652–662. https://doi.org/10.1177/1076029612454938
- Quinones-Hinojosa, A., Gulati, M., Singh, V., & Lawton, M. T. (2003). Spontaneous intracerebral hemorrhage due to coagulation disorders. Neurosurgical Focus, 15(4), E3. https://doi.org/10.3171/foc.2003.15.4.3
- Tsatsakis, A., Docea, A. O., Calina, D., Tsarouhas, K., Zamfira, L.-M., Mitrut, R., Sharifi-Rad, J., Kovatsi, L., Siokas, V., Dardiotis, E., Drakoulis, N., Lazopoulos, G., Tsitsimpikou, C., Mitsias, P., & Neagu, M. (2019). A Mechanistic and Pathophysiological Approach for Stroke Associated with Drugs of Abuse. Journal of Clinical Medicine, 8(9). https://doi.org/10.3390/jcm8091295
- Bos, M. J., Koudstaal, P. J., Hofman, A., & Breteler, M. M. B. (2007). Decreased glomerular filtration rate is a risk factor for hemorrhagic but not for ischemic stroke: the Rotterdam Study. Stroke, 38(12), 3127–3132. https://doi.org/10.1161/STROKEAHA.107.489807
- Chen, Y.-C., Chang, K.-H., & Chen, C.-M. (2018). Genetic Polymorphisms Associated with Spontaneous Intracerebral Hemorrhage. International Journal of Molecular Sciences, 19(12). https://doi.org/10.3390/ijms19123879
- Aguilar, M. I., & Brott, T. G. (2011). Update in intracerebral hemorrhage. The Neurohospitalist, 1(3), 148–159. https://doi.org/10.1177/1941875211409050
- Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 42228. (n.d.-b). lobar-haemorrhage-1. https://radiopaedia.org/articles/lobar-haemorrhage
- Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 10224. (n.d.-a). pontine hemorrhage. https://radiopaedia.org/articles/pontine-haemorrhage?lang=us
- Zheng, H., Chen, C., Zhang, J., & Hu, Z. (2016). Mechanism and Therapy of Brain Edema after Intracerebral Hemorrhage. Cerebrovascular Diseases (Basel, Switzerland), 42(3–4), 155–169. https://doi.org/10.1159/000445170
- complications of ICH, Minneapolis clinic of neurology. (n.d.). https://minneapolisclinic.com/patient-resources/intracerebral-hemorrhage-hemorrhagic-stroke/
- Case courtesy of Dr David Cuete, Radiopaedia.org, rID: 27193. (n.d.). cerebellar-haemorrhage and vasogenic edema. https://radiopaedia.org/articles/intracerebral-haemorrhage?lang=us
- Doria, J. W., & Forgacs, P. B. (2019). Incidence, Implications, and Management of Seizures Following Ischemic and Hemorrhagic Stroke. Current Neurology and Neuroscience Reports, 19(7), 37. https://doi.org/10.1007/s11910-019-0957-4
- Lord, A. S., Langefeld, C. D., Sekar, P., Moomaw, C. J., Badjatia, N., Vashkevich, A., Rosand, J., Osborne, J., Woo, D., & Elkind, M. S. V. (2014). Infection after intracerebral hemorrhage: risk factors and association with outcomes in the ethnic/racial variations of intracerebral hemorrhage study. Stroke, 45(12), 3535–3542. https://doi.org/10.1161/STROKEAHA.114.006435
- de Freitas, G. R., & Nagayama, M. (2009). Deep venous thrombosis after intracerebral hemorrhage, gender and ethnicity: a challenge for therapeutic approaches. Cerebrovascular Diseases (Basel, Switzerland), 27(4), 320–321. https://doi.org/10.1159/000202007
- Macellari, F., Paciaroni, M., Agnelli, G., & Caso, V. (2014). Neuroimaging in intracerebral hemorrhage. Stroke, 45(3), 903–908. https://doi.org/10.1161/STROKEAHA.113.003701
- Case courtesy of Dr Ian Bickle, Radiopaedia.org, rID: 76781. (n.d.). frontal-lobe-haematoma. https://radiopaedia.org/cases/frontal-lobe-haematoma
- Dr Rohit Sharma and Dr Evyn Arnfield et al. (n.d.). ABC/2. https://radiopaedia.org/articles/abc2
- Case courtesy of Dr Mohammad Farghali Ali Tosson, Radiopaedia.org, rID: 52302. (n.d.). intracranial-haemorrhage-with-blood-fluid level. https://radiopaedia.org/cases/intracranial-haemorrhage-with-fluid-fluid-level-1
- Case courtesy of Dr Jeremy Jones, Radiopaedia.org, rID: 6223. (n.d.). intracerebral-haemorrhage-with-ventricular-extension. https://radiopaedia.org/cases/intracerebral-haemorrhage-with-ventricular-extension?lang=us
- Case courtesy of Dr Stefan Ludwig, Radiopaedia.org, rID: 13919. (n.d.). cerebral-arteriovenous-malformation. https://radiopaedia.org/articles/cerebral-arteriovenous-malformation
- Case courtesy of A/Professor Christen Barras, Radiopaedia.org, rID: 24481. (n.d.). intracranial-haemorrhage-cta-spot-sign. https://radiopaedia.org/articles/ct-angiographic-spot-sign-intracerebral-haemorrhage?lang=us
- Al, D. D. J. B. and A. P. F. G. et. (n.d.). hemorrhage on MRI. https://radiopaedia.org/articles/haemorrhage-on-mri
- Case courtesy of Dr Ahmed Abdrabou, Radiopaedia.org, rID: 22749. (n.d.). hypertensive-intracerebral-haemorrhage T1. https://prod-images-static.radiopaedia.org/images/3251974/11274cdbb5fa0a305ab53bdf31ecc8_big_gallery.jpg
- Case courtesy of Dr Ahmed Abdrabou, Radiopaedia.org, rID: 22749. (n.d.-b). hypertensive-intracerebral-haemorrhage T2. https://prod-images-static.radiopaedia.org/images/3252272/8529f95080ca7ef6f3cdc52aa00916_big_gallery.jpg
- Case courtesy of Dr Bita Abbasi, Radiopaedia.org, rID: 22709. (n.d.). cerebral-arteriovenous-MRI. https://radiopaedia.org/articles/cerebral-arteriovenous-malformation
- Case courtesy of Dr Abdulrahman Abdo Ali Abbas , Radiopaedia.org, rID: 78910. (n.d.). hemorrhagic-cystic-glioblastoma. https://radiopaedia.org/articles/haemorrhagic-intracranial-tumours
- Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 4564. (n.d.-c). chronic-hypertensive-encephalopathy. https://radiopaedia.org/articles/cerebral-microhaemorrhage
- Hemphill, J. C. 3rd, Greenberg, S. M., Anderson, C. S., Becker, K., Bendok, B. R., Cushman, M., Fung, G. L., Goldstein, J. N., Macdonald, R. L., Mitchell, P. H., Scott, P. A., Selim, M. H., & Woo, D. (2015). Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 46(7), 2032–2060. https://doi.org/10.1161/STR.0000000000000069
- Morotti, A., & Goldstein, J. N. (2016). Diagnosis and Management of Acute Intracerebral Hemorrhage. Emergency Medicine Clinics of North America, 34(4), 883–899. https://doi.org/10.1016/j.emc.2016.06.010
- Stanton, R. J., Eckman, M. H., Woo, D., Moomaw, C. J., Haverbusch, M., Flaherty, M. L., & Kleindorfer, D. O. (2020). Ischemic Stroke and Bleeding: Clinical Benefit of Anticoagulation in Atrial Fibrillation After Intracerebral Hemorrhage. Stroke, 51(3), 808–814. https://doi.org/10.1161/STROKEAHA.119.027370
- Bhattathiri, P. S., Gregson, B., Prasad, K. S. M., & Mendelow, A. D. (2006). Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochirurgica. Supplement, 96, 65–68. https://doi.org/10.1007/3-211-30714-1_16
- Al-Mufti, F., Thabet, A. M., Singh, T., El-Ghanem, M., Amuluru, K., & Gandhi, C. D. (2018). Clinical and Radiographic Predictors of Intracerebral Hemorrhage Outcome. Interventional Neurology, 7(1–2), 118–136. https://doi.org/10.1159/000484571
- Saxena, A., Anderson, C. S., Wang, X., Sato, S., Arima, H., Chan, E., Muñoz-Venturelli, P., Delcourt, C., Robinson, T., Stapf, C., Lavados, P. M., Wang, J., Neal, B., Chalmers, J., & Heeley, E. (2016). Prognostic Significance of Hyperglycemia in Acute Intracerebral Hemorrhage: The INTERACT2 Study. Stroke, 47(3), 682–688. https://doi.org/10.1161/STROKEAHA.115.011627
- Diener, H.-C., & Hankey, G. J. (2020). Primary and Secondary Prevention of Ischemic Stroke and Cerebral Hemorrhage: JACC Focus Seminar. Journal of the American College of Cardiology, 75(15), 1804–1818. https://doi.org/10.1016/j.jacc.2019.12.072
- Dogra, S., Jain, R., Cao, M., Bilaloglu, S., Zagzag, D., Hochman, S., Lewis, A., Melmed, K., Hochman, K., Horwitz, L., Galetta, S., & Berger, J. (2020). Hemorrhagic stroke and anticoagulation in COVID-19. Journal of Stroke and Cerebrovascular Diseases : The Official Journal of National Stroke Association, 29(8), 104984. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.104984
- Ji, R., Li, G., Zhang, R., Hou, H., Zhao, X., & Wang, Y. (2019). Higher risk of deep vein thrombosis after hemorrhagic stroke than after acute ischemic stroke. Journal of Vascular Nursing : Official Publication of the Society for Peripheral Vascular Nursing, 37(1), 18–27. https://doi.org/10.1016/j.jvn.2018.10.006
- Mengel, A., Stefanou, M.-I., Hadaschik, K. A., Wolf, M., Stadler, V., Poli, K., Lindig, T., Ernemann, U., Grimm, F., Tatagiba, M., Ziemann, U., & Poli, S. (2020). Early Administration of Desmopressin and Platelet Transfusion for Reducing Hematoma Expansion in Patients With Acute Antiplatelet Therapy Associated Intracerebral Hemorrhage. Critical Care Medicine, 48(7), 1009–1017. https://doi.org/10.1097/CCM.0000000000004348
- Chaucer, B., Demanes, A., Stone, A., & Kakollu, V. (2020). Hemorrhagic Stroke in Relapsing Polychondritis: A Rare Complication of a Rare Disease. In Case reports in rheumatology (Vol. 2020, p. 7464503). https://doi.org/10.1155/2020/7464503