Article title: Cerebral Vasospasm
Author: Mahmoud Algharabli
Scientific Editor: Dr Omar Jbarah
Linguistic Editor: Philip Sweidan
HISTORY OF CEREBRAL VASOSPASM
Cerebral vasospasm was firstly described by an English physician called sir William’s gull in 1859. He had published a case report of Mrs. Fanny S—, a 30-year-old female patient. It had all started with a subarachnoid hemorrhage (SAH) in November 4,1850 that involved transient loss of consciousness, vomiting and nausea. She was admitted to the hospital on the next day comatosed with right hemiplegia. On the third day of admission, she had regained her consciousness and was able to eat. On the fourth day of admission, she was able to recognize a family member like her “cousin”. However, on the fifth day of admission, she had developed progressive neurological deterioration and died 6 days post SAH. At autopsy, a large middle cerebral artery (MCA) aneurysm rupture was recognized close to a second unruptured aneurysm in a background of large clot in the sylvian fissure and there was softening in many areas of the brain. The findings that were discovered at autopsy and the timeline of the signs and symptoms of the patient are all directing to cerebral infarction due to cerebral vasospasm, although it wasn’t known at that time.(1) Since sir William first description about cerebral vasospasm, many investigators to date are studying this phenomenon to understand its pathophysiology and causes.
Introduction:
Cerebral vasospasm is a phenomenon that can be defined in 2 ways, a clinical or an angiographic definition. Angiographic cerebral vasospasm is defined as the narrowing of the dye column seen in major cerebral arteries that is often focal but could be diffuse. This narrowing depends mainly on the timeline of the SAH which is rarely pronounced before day 4 of the initial hemorrhage and reach the peak at day 7. At that window of time, 40 to 60% will have some reduction in the diameter of the one or more of the arteries of circle of willis. (2-13) On the other hand, clinical vasospasm is defined as the consequences of cerebral arterial narrowing and is marked by the insidious onset of confusion and decreased level of consciousness followed by motor and sensory focal deficits. Although, clinical vasospasm usually parallels with the presence of angiographic vasospasm, there are 70% of patients that may develop angiographic narrowing of arteries and only 20% to 30% will manifest neurological deficits. (14-17) The contradiction between the incidence of angiographic and clinical vasospasm is probably best understood by the differences in the location and degree of arterial narrowing and the adequacy of collateral circulation. There are studies reporting that the timeline onset of vasospasm occurs at least 3 days after the aneurysmal rupture of arteries and usually parallels with clinical deterioration. It was found that Vessel narrowing peak at 5 to 14 days and gradually resolves within 2 to 4 weeks.(18-20), a term called delayed cerebral ischemia (DCI) which is defined as neurological deterioration presumed to be related to reduced blood supply that lasts for more than an hour and does not have an identifiable cause.(21)
As a consequence of this, arterial narrowing became accepted as the underlying mechanism of DCI. Although Cerebral vasospasm mainly occur due to aneurysmal SAH in clinical practice, there are reasons that may lead to it as a rare consequence of traumatic subarachnoid hemorrhage (SAH), ruptured vascular malformations, hemorrhagic brain tumors, and for sure any condition that results in extensive bleeding into the subarachnoid space beneath the brain.
Epidemiology, etiology, pathophysiology, diagnosis and different types of cerebral vasospasm prevention and treatments will be discussed in this article.
Epidemiology:
The incidence and prevalence of cerebral vasospasm is strongly correlated to aSAH as it is the main reason. It was estimated that 30.000 of north American patients experience aneurysmal subarachnoid hemorrhage (aSAH) each year and of these 12-30% has been estimated to get cerebral vasospasm. (22-23) 50% of patients suffering from aSAH die before reaching medical care. Of those who reach an acceptable neurosurgical center and have their aSAH treated, an estimated 14% die or sustain a significant morbidity due to cerebral vasospasm. (23) Overall incidence of Angiographic vasospasm after rupture of an aneurysm is from 50% to 90%. (24)
Predication of vasospasm:
Vasosapasm can be predicated in patients after SAH based on CT-finding of the initial hemorrhage and non-CT predicative risk factors. C. Miller Fisher was the first one to develop a grading scale to assess the risk of developing vasospasm based on CT-findings in 1980. He had concluded that a localized clot or diffuse blood greater than 1 mm was highly predicative risk factor of developing vasospasm.(25) The scale was criticized greatly and many modifications has been made on it due to lack in difference between grade 1,2 in the clinical picture with grade 3,4 and the presence of interventricular bleeding (IVB) with thick cisternal clots doesn’t change the patient clinical picture.(26-29) As a a matter of fact, a modified fisher grade had been developed by n, Frontera et al which it was found as a good predictive of symptomatic vasospasm (not DCI).(30-31) “clarifying figures”
Figure 1: The Modified Fisher CT rating scale: Grade 1 (minimal or diffuse thin SAH without IVH), indicating low risk for symptomatic vasospasm; Grade 2 (minimal or thin SAH with IVH) and Grade 3 (thick cisternal clot without IVH), indicating intermediate risk for symptomatic vasospasm; and Grade 4 (cisternal clot with IVH), indicating high risk for symptomatic vasospasm. Reproduced with permission from Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, Connolly ES Jr, Mayer SA: Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 32:2012-2020, 2001. From: Frontera: Neurosurgery, Volume 59(1).July 2006.21-27(30-31)

Figure 2: the difference between the classical and modified fisher scales(30-31)
-Non-CT-findings predicative of vasospasm:
They include loss of consciousness (LOC) at the time of rupture, smoking, diabetes mellitus (DM), hyperglycemia or bad neurological grade on admission. (32-34) The location of the ruptured aneurysm as distal anterior cerebral aneurysm also possesses a high incidence of symptomatic vasospasm. (35)
Etiology and pathogenesis:
As we discussed above, the etiology of cerebral vasospasm has many reasons but are rare except aSAH. This allowed us to explore the pathogenesis of cerebral vasospasm in the background of aSAH. The pathogenesis of cerebral vasospasm is poorly understood. The uncertainty of vasospasm mechanism is mainly due to the controversy of histopathological changes in the involved arteries as it was reported that the morphology of narrowed arteries are normal following experimental SAH (36), in comparison to the microscopic alterations that occur in vasospasm which we have few information about them. Many changes have been described such as: platelet adhesion within the lumen and aggregation with white blood cells adhesion and thrombus formation, (37-38) increased endothelial pinocytotic activity or channel formation, intimal swelling, proliferation, degeneration, and desquamation also opening of interendothelial junctions (39-44) and many more. There was no conclusive evidence forthcoming to indicate whether or not any of these changes are direct causes to the arterial narrowing and development of neurological deficits. Furthermore, to date there is a better perception that blood in the subarachnoid space is related to the development of vasospasm and that there is a very tight association between vasospasm and the amount of blood in the subarachnoid space. (45-51) The bleeding into the subarachnoid space appears to be the core initiating event and knowledge of specific spasmogenic substances released from the clot has not been accomplished definitively. (52) Spasmogens supposed to be released include epinephrine, norepinephrine and angiotensin (53-60). Furthermore, it must be noted that subarachnoid hemorrhage is associated with a sudden increase in intracranial pressure (ICP) and blood pressure with some degree of hypoxia and cerebral ischemia as these factors are implicated in development of vasospasm. (61-64) However, the most major modern hypothesis concerning about arterial narrowing is as follows:
1) cerebral arterial smooth muscle cells contraction due to vasoactive substances found in the CSF, (52, 65-68) denervation exaggerated sensitivity (69-71),72,73 or a prostacyclin/thromboxane A2 imbalance.(74-76)
2) Abnormalities of vasodilatory activity due to firstly, prostacyclin/thromboxane A2 imbalance. Arterial wall prostaglandins amount declines progressively following SAH, and this is probably in association with the progressive endothelial degeneration. (74-76) second of all, hemorrhagic CSF has Oxyhemoglobin which has been shown to inhibit acetylcholine-mediated vasodilatation. (77) This acetylcholine-mediated vasodilatation is inhibited by hypoxia from hemorrhage (78) and the arterial adventitial pores may get clogged by fibrin and red blood cells which also contributes to arterial muscle cell hypoxia.(79) Furthermore, the hemoglobin (Hgb) found in the CSF may suppress neurogenic vasodilatation,(80) and it can also inhibit endothelial derived relaxing factor-induced vasodilatation (Fujiwara S, Kassell NF, unpublished data).
3) Proliferative vasculopathy due to Mitogenic substances found in the thrombocytes which is either found in the subarachnoid clot or those that stuck to the arterial lumen, also the injured endothelium release a substance that is similar to those mitogenic factors which may cause proliferation of smooth muscle cells and fibroblasts in the media. (81-82)
4) Vasospasm may be in a part due to an underlying immunoreactive process as it has been shown that IgG and C3 deposits in the medial layer of the arterial wall of patients with chronic spasm. (83) It has been evidenced that there is an increase in circulating immune complexes. (84)
5) Vasospasm may be an inflammatory process. There was a considerable amount of indirect evidence proposing that vasospasm is a result of inflammatory process taking place in the arterial wall which is also initiated by the surrounding clot.(84-88) There are multiple morphological changes congruent with an inflammatory process (87, 88 ,89) as there are leukocytes within the blood vessel wall (91) or stuck to the endothelial surface (Nazar G, Kassell NF, unpublished data), in addition to the experimental responses to nonsteroidal anti-inflammatory agents (NSAIDs) that back up the concept.(92-93)
6) Mechanical phenomena is a possible cause of arterial narrowing as it could be compressed either by periarterial clot or due to deformation of the arachnoid bands which tether the major cerebral arteries in the subarachnoid cisterns. (94)
It is obvious that the above hypotheses are pointing toward the substances liberated from the subarachnoid clot as the cause of the development of arterial narrowing.
Recently, it has been proposed that damaged endothelium resulting from the SAH may be linked to the arterial narrowing. (95) The underlying mechanism of endothelial damage has not been established but arterial hypertension, increased intracranial pressure and specifically subarachnoid blood all increase transendothelial permeability acutely, either by achieving a higher degree of vesicular transport or channel formation (Sasaki T, Kassell NF, unpublished data). Clinically, contrast enhancement was observed in the basal cisterns where the major cerebral arteries are found indicating increased arterial permeability occurring in certain patients following aneurysmal rupture and these patients are most prone to develop vasospasm. (96-99) In further stages of SAH as subacute or chronic, the endothelial cells of arteries degenerate and there will be a gap of the interendothelial tight junctions which will result in disturbance of blood arterial wall barrier.(95) This disturbance of barrier may permit the access of several constrictor substance as catecholamines or prostaglandins to inside smooth muscle cells. Furthermore, platelets may release additional constrictor substances as serotonin when they adhere to either disrupted endothelium or naked subendothelium, (100-101) also it has been evident that there is depolarization of arterial smooth cells caused by subarachnoid hemorrhage making them in a risk of contraction. (102) In addition, damaged endothelium may lead to loss of very strong vasodilators as prostacyclin, (66-68) while the secretion of thromboxane A2 from platelets is continuously unopposed. Moreover, adhering platelets and white blood cells may additively release some vasoactive substances (100-101,103-104). Platelet derived growth factor (PDGF) secreted either from platelets found in the clot of SAH or platelets found stuck on the luminal surface of the arterial wall or related substances released from damaged endothelium, (105-106) or the effects of thrombin, plasmin and fibrinogens spasminogens (107) which all results in proliferation of smooth muscles cells and fibroblasts which may lead to medial thickening and luminal narrowing of arteries.
As a summary, of the above discussion it can be stated that the pathogenesis of cerebral vasospasm is very complicated, not clearly understood and a multifactorial process.
Clinical presentation:
As we discussed previously, vasospasm has multiple terminologies as DCI, clinical and angiographic vasospasm and our concern now is symptomatic vasospasm and delayed cerebral ischemia not angiographic vasospasm.
As we discussed, vasospasm rarely starts before day 3 from the initial insult and its peak is at 7 days so if it occurs and had symptoms it is called clinical cerebral vasospasm. It typically refers to the clinical worsening of symptoms after the initial SAH. A Japanese study discovered through a survey that symptoms were mainly focal deficits coming at first followed by motor paresis then decline in the level of consciousness and no other cause for the neurological worsening can be noticed except cerebral vasospasm in addition to presence for worsening headache, low-grade fever, elevation of blood pressure, (108) in the context of exclusion other possible causes of deterioration as hydrocephalus, seizures, metabolic derangement, infection or oversedation. Symptomatic vasospasm is a subjective diagnosis which can be limited to poor prognosis cases in which neurological examination can be difficult as it could be subtle. (109) Delayed cerebral ischemia is known as symptomatic vasospasm, infarction attributable to vasospasm, or both. (110)
Diagnosis:
Detection and monitoring of cerebral vasospasm after aSAH is necessary as both cerebral vasospasm (CV) and delayed cerebral ischemia have to be prevented as possible and if they occur then it should be treated as soon as possible (111). Asymptomatic arterial narrowing is extremely frequent after SAH and CV becomes symptomatic only in about 30 % of patients. (112-113) Detection of CV is not based on standardized criteria also neither are the exams to assess related hypoperfusion and ischemia. (114) The following methods are most commonly used in diagnosing cerebral vasospasm:
- Transcranial Doppler (TCD) ultrasound :
TCD ultrasound is considered the main non-invasive diagnostic tool of cerebral vasospasm. (115) Despite variable sensitivity and specificity of TCD, it has class A, level 2 evidence supporting its use in a reliable way in diagnosing cerebral vasospasm. (116) The underlying mechanism of TCD is when the artery narrows, blood flow velocity within it gets high, causing a doppler shift frequency between emitted and reflected waves. (117) It is used frequently to monitor patients with SAH for increases in intracranial blood flow velocity which could be sign of imminent vasospasm. The TCD consists of a 2 MHz ultrasound probe whereas a probe can be fixed in headset or manually applied on the acoustic windows which is all dependent on the duration of the procedure. Ultrasound waves need to be transmitted to the cerebral circulation and this can be done through acoustic windows which are regions of the skull either thin bone or foramina and they include the transtemporal, transorbital, suboccipital and submandibular windows. (118)
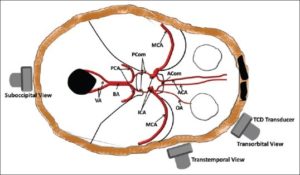
Figure 3: Acoustic windows for insonation of cerebral arteries (118)
Detection of proximal cerebral vasospasm by TCD is excellent as there are segmental and diffuse increases in blood flow velocity in these vessels compared to more distal vessels. (117) Factors such as blood pressure and cerebral blood flow can affect the velocity so distinguishing between vasospastic and hyperemic increases in blood velocity have been established to be facilitated by measuring cervical internal carotid artery (ICA) velocity and intracranial blood velocity. Furthermore, a good association between TCD velocities and vasospasm was established, as velocities in middle cerebral artery (MCA) greater than 120cm/sec are indicative of some degree of vasospasm and those more than 200cm/sec is strongly correlated with severe vasospasm. (119) There are parameters such as Lindegaard ratio (LR), SVIRI ratio and mean flow velocity which all are derived from TCD. The LR can be taken by dividing the mean flow velocity of MCA by those of ipsilateral cranial ICA. The SVIRI ratio can be taken by dividing mean flow velocities of basilar artery (BA) by those of extracranial vertebral artery (VA). (120-121) LR greater than 3 is consistent with vasospasm but in hyperemia the ratio is the same as there is an increase in velocities in both MCA and ICA. (117,119) Utilization of TCD is best when MCA values are clearly low (less than 120cm/sec or very high as more than 200cm/sec), and the respective negative and positive predicative values for significant angiographic vasospasm will reach 90%. (122-123) In distal branches (peripheral) TCD is not preferably used as it is unreliable, (124) which could be the reason for its failure in correlating with perfusion deficits detected on cerebral blood flow (CBF) studies. (125) Based on the above parameters (ratios) vasospasm can be classified into mild moderate and severe and the table will demonstrate it.
| Degree of MCA vasospasm (121) | Mean flow velocity(cm/sec) | LR |
| mild | 120-149 | 3-6 |
| Moderate | 150-199 | 3-6 |
| severe | More than 200 | More than 9 |
| Degree of BA vasospasm (126) | Mean flow velocity(cm/sec) | SVIRI ratio |
| Presence of vasospasm | More than 70 | More than 2 |
| Moderate or severe | More than 85 | More than 2.5 |
| severe | More than 85 | More than 3 |
TCD ultrasonography done by skilled technicians is considered useful in many neurosurgical units in surveillance of SAH, but the results must be individualized along with all other information available.
- Vascular imaging:
The main imagining modalities of medium and large cerebral arteries are computed tomography angiograph (CTA) and digital subtraction angiography (DSA). (127) Magnetic resonance imaging is also considered a good tool in terms of the information it provides but it is difficult to be done on critically ill-patient. (128)
–CTA is considered a reliable and accurate tool in terms of illustrating the anatomy of intracranial vessels and provides the possibility of rapid diagnosis and monitoring of CV. (129) It is considered the initial screening test which had shown in 10 recent studies that CTA had a cumulative sensitivity and specificity of 80% and 93% respectively but it had failed to be more accurate than DSA. (130) However, computed tomography perfusion (CTP) scans recently have been proved to have more diagnostic utility than CTA. (131) Utilization of an intuitive visual grading scale as: non, mild (less than 30% narrowing), moderate (between 30% and 60%) and severe (more than 60%) can be used to quantify CV based on each proximal vessel of circle of Willis till its secondary branches. (132) CTA interpretation of CV can be limited by the clips and coils used in aneurysmal treatment as they produce artifact. This limitation can be reduced by increasing clip-gantry angle and the use of titanium clips rather than cobalt alloy clips which will reduce the artifact. (133) On admission angiography, early vasospasm within 48 hours of aneurysmal rupture has been noticed in a small percentage of patients with SAH, however without a baseline angiography it is not clear how this diagnosis can be made accurately in all cases. (134) In order to correctly evaluate the degree of CV and to prevent the bias of vessel hypoplasia, each narrowed arterial segment should be compared with the same segment shown in the previous baseline CTA or even DSA (if it was done at admission for aneurysmal diagnosis and/or early after aneurysm treatment). (132)
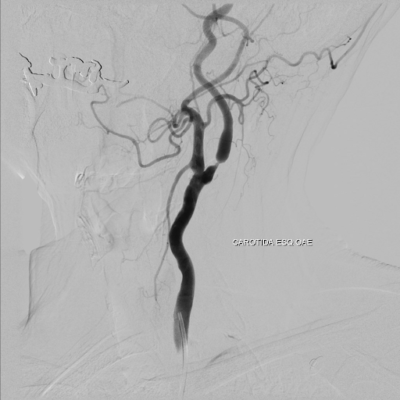
-DSA: It is considered the gold standard for diagnosing CV mainly the 2-dimensional DSA (2d-DSA). (133-134) It allows accurate quantitively assessment of every intracranial artery and it allows for the possibility of endovascular intervention if needed. (135-136) However, because its invasive nature as a procedure and the is risk of developing complications related to the angiography in 1.3–2.4 % of patients in which about 0.5 % these deficits can be permanent. (137-138) Due to its difficulty as it is invasive and it could be complicated, it is necessary to limit angiography only to selected cases to confirm diagnosis and treat resistance to medical therapy.
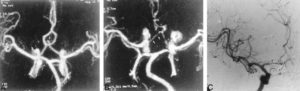
Figure 4: 56-year-old woman with SAH. A, Preoperative MIP image from a CTA study shows an anterior communicating artery aneurysm (arrow). B, Postoperative CTA shows clipping of the aneurysm and narrowing of the anterior cerebral artery (arrows), consistent with vasospasm.C, DSA, anteroposterior view, confirms vasospasm involving the A1 segment of the anterior cerebral artery (arrows). (139)
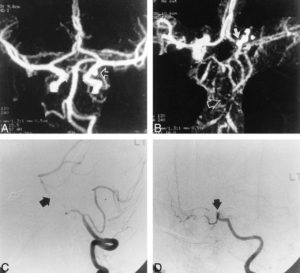
Figure 5: 28-year-old man with an aneurysmal SAH. A, Preoperative CTA, MIP image, reveals a posterior communicating artery aneurysm (arrow). B, Postoperative CTA shows severe basilar artery spasm (open arrow) and carotid artery occlusion (solid arrow). C, DSA, lateral view, confirms basilar arteryvasospasm (arrow). D, DSA, lateral oblique view, verifies supraclinoid ICA occlusion (arrow). (139)
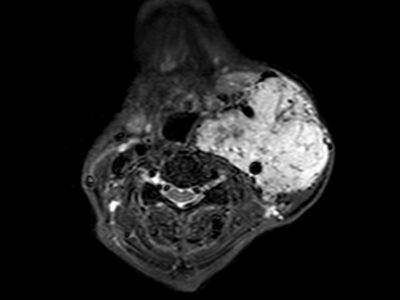
-MRI for CV: Perfusion-weighted MRI has been shown to reveal small regions of early ischemic injury, which indicate areas suffering from vasospasm. (140) Diffusion weighed MRI shows Decreases in relative cerebral blood volume (CBV) and mean transit time that help in the selection of patients who benefit from triple-H therapy. (141) On the other hand, Magnetic Resonance Angiography (MRA) was initially used for the diagnosis of intracranial aneurysms and vascular stenosis associated with atherosclerosis, it wasn’t routinely applied to the diagnosis of vasospasm until 1995. (142) MRA may now be performed using various flow-independent strategies and is has the ability of addressing both radiographic vessel narrowing and physiological measurements related to vasospasm. (143-144)
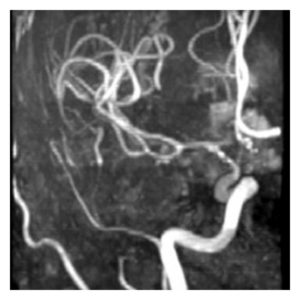
Figure 6: MRA, Vasospasm of the supraclinoid left internal carotid artery, middle cerebral artery, and anterior cerebral artery demonstrated on time of flight MRA. (145)
- Cerebral blood flow and perfusion measurements:
there are several modalities for examining cerebral blood flow (CBP) and perfusion. Many of them are old as, positron emission tomography (PET), xenon-enhanced CT (Xe-CT), and single-photon emission computed tomography (SPECT) which are used in evaluation of brain tissue distal to vessels undergoing vasospasm. (146)
-Xe-CT: this method works based on the administration of intravenous or inhalation of radioactive xenon which is a radiopaque diffusible gas that dissolves quickly in the blood after inhalation and diffuses rapidly into the parenchyma according to blood flow. Xe-CT provides only snapshots over time and measures only the CBF. (147)
-SPECT: a nuclear imaging tool that measures CBF by detecting flow-dependent uptake of a radioactive tracer which is commonly a technetium-99m coupled to hexamethyl propyleneamine oxime. (148) This accumulates in the brain parenchyma proportionally to blood flow, particularly during first pass through the microcirculation. (149) Thus, parenchymal flow reduction is noticed independently from its etiology (proximal and or distal CV, brain oedema, hypotension, etc.). Through this diagnostic tool, the entire brain can be visualized, although resolution is relatively low. In comparison to DSA the Sensitivity and specificity for CV are 89 and 75 %, respectively. (150)
-PET: a nuclear medicine technique which provides quantitative hemodynamic measures including CBF, CBV, OXYGEN extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2). Tracers include several oxygens (O2) bound isotopes as an example for the isotopes are, 15O-labeled water (151-152) to measure CBF, 15O-labeled carbon monoxide (153) to measure CBV and 15O-labeled oxygen to measure regional OEF. (154)
-CTP scan: a tool that measures the CBF by studying the cerebral flow dynamic (155) and it works based on a helical CT scanner with an appropriate postprocessing software. It has been used in patients with SAH (156,157) and it is increasingly becoming more popular due to its ease of use, speed of image acquisition and good correlation with DSA for angiographic vasospasm. (158) Angiographic vasospasm discovered in patients with 50% and more narrowing are prone to have perfusion deficits with reduced CBF (159), and the degree of angiographic vasospasm correlates with the degree of perfusion impairment as the vascular areas of the spastic vessels is proportional to the more hypoperfused regions. (160) CTP can early detect the presence of reduced CBF, delay in mean transit time which are indicative of ischemia, and the status of cerebral blood volume, which is normally maintained in penumbral brain but is markedly decreased in established infarcts. (161) The sensitivity of CTP In patients with confirmed angiographic vasospasm is 74% and specificity is 93% found in recent meta-analysis. (162)
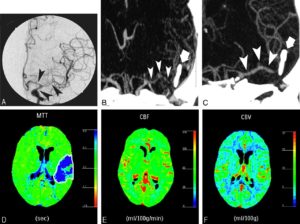
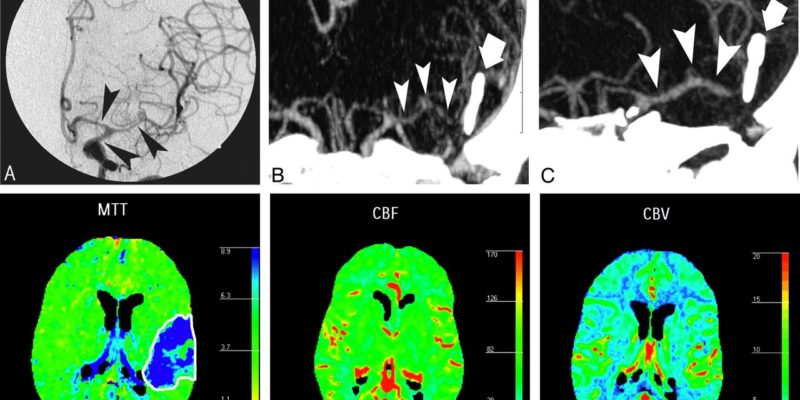
Figure 7: A 44-year-old man with right-sided hemiparesis attributed to cerebrovascular vasospasm occurring 9 days after SAH consecutive to a ruptured aneurysm of the anterior communicating artery, treated by surgical clipping. DSA (A) showed moderate vasospasm on M1 segment of left MCA and focal severe vasospasm on the M2 segment (arrowheads). These findings were confirmed by MIP MSCTA reconstruction (B) and volume-rendered MSCTA reconstruction (C). Perfusion CT performed during the same CT session revealed an increase in MTT (D) and a decrease in rCBF (E), with slight increase of rCBV (F). This pattern of perfusion alterations corresponds to a reversible ischemic lesion consecutive to vasospasm. The patient was then treated by a local intra-arterial nimodipine infusion and a balloon angioplasty of the left M1 segment.(163)
- Invasive Tissue Monitoring:
It includes Brain Tissue Oxygen Monitoring and Microdialysis. Brain tissue oxygen monitoring (ptiO2) of SAH showed low cerebral tissue Ph and higher pco2 compared to controls in 2000 the first time it was used (164), and it was evident by subsequent investigation that there is a strong correlation between PtiO2 and vasospasm. (165) There is a separate study which suggested that PtiO2 pressure reactivity is a reliable indicator of impaired autoregulation and, by warrant, vasospasm effect. (166) There was a questioning about the utility and reliably of Ptio2 as it was explored by some that the relationship between pressure- and oxygen-related indices of vasospasm and clinical outcome had showed a disproportionate relationship. (167) On the other hand, Cerebral microdialysis is a tool that is capable of measuring local levels of different metabolic markers including glutamate (168–170), lactate (168, 171) and glucose (171) and correlating these values to areas of ischemia and clinical outcome. Microdialysis has been shown to be by 89% specific for ischemia, through the capability of detecting huge changes in concentrations of lactate, glucose, and glutamate levels before a patient becomes symptomatic in a specific study. (172) Higher levels of lactate, nitrite, and taurine was associated with poor neurologic outcome after SAH and all of them was measured by microdialysis. (173) Microdialysis by its nature is an invasive tool so it carries risks of cerebral hematoma and infection, along with the possibility of probe malfunctioning or misplacement. (174)
- Near infrared specterscopy (NIRS):
A tool that may prove to be useful for bedside monitoring of cerebral ischemia following SAH as it is simple, non-invasive tool. (175-177) It works by measuring changes in deoxyhemoglobin concentration in cortical vessels.(178-179) Quantitative assessment of hemoglobin concentration can be done by specifically time resolved NIRS and in one pilot study it was found to be more sensitive than TCD in diagnosis of CV. (180) NIRS detect decline of cortical oxygen saturation, total hemoglobin and oxyhemoglobin during CV. (181) Despite some limits as NIRS measures blood oxygenation predominantly in cortical venous blood in specific cortical region in addition to the CSF, the skull, and the other layers of the human head can interfere with the photon signals.(182) Eventullay, NIRS provides a continuous and noninvasive metabolic monitoring which can be useful in multimodal approach to management.
Prevention:
Over the past few decades, many methods of preventing vasospasm and subsequent DCI were examined but no major breakthroughs in complete prevention have been identified. In the following, we will discuss the most common preventive methods used.
- General preventive methods and fluid management:
In the acute setting of SAH, patients tend to have volume contraction, (183) and hypovolemia should be avoided. Intentional induction of Hypervolemia and volume expansion therapy is not obviously clear that it is useful in terms of prevention of vasospasm or ischemia, or even possible in patients with normal renal function. (184-187) Hydration with 3L of isotonic fluids should be done for all patients daily and there is some evidence that additional colloid infusions such as albumin are beneficial. (188) Some patients with SAH may experience excessive natriuresis, known as cerebral salt wasting which is usually related to elevations in brain natriuretic peptide, and they are susceptible to the development of hyponatremia during this time. There may be an increase in the risk for vasospasm (189) in addition there is an association of cerebral infarction in poor grade patients with hyponatremia. (190) As a result of those facts, hyponatremia in the setting of SAH should be treated with salt replacement in the form of normal or 3% hypertonic saline, combined with fludrocortisone (Florinef) administration (0.3 mg/ day) if the patient is experiencing vasospasm not with fluid restriction. (190,191) Cerebral perfusion pressure (CPP) is very important parameter to monitor and maintain at 70 mm Hg or greater in poor-grade patients in patients with external ventricular drains.(192) Furthermore, perfect ventilation and oxygenation, prevention of fever, maintenance of euglycemia and good nutrition, and attention to electrolytes especially sodium is important in reduction the effects of vasospasm and delayed ischemia.
- Calcium channel blockers(CCB):
In terms of prevention of secondary ischemia, CCB was rationally introduced due to its mechanism of blocking the dihydropyridine-type calcium channel, thereby preventing the influx of calcium into the vascular smooth-muscle cells and decreasing the rate of cerebral vasospasm. (193) Nimodipine is considered the only drug that showed decreased cerebral ischemia and improved outcomes in patients with SAH when it is given within 72 hours of presentation. Furthermore, the British aneurysm nimodipine trial that was published in 1989 remains the only drug treatment regimen that has class 1A evidence of efficacy. The study was a double-blind, placebo-controlled trial that randomized 554 patients presenting with SAH to receive either placebo or nimodipine 60 mg every 4 hours, starting within 96 h of presentation. The therapy was for a whole 21 days. At 3 months duration the 2 groups were compared for rates of infarction and poor outcome, as defined by the Glasgow Outcome Scale (GOS) of 1–3 (dead, vegetative state, or severe disability); and rebleeding. In patients given nimodipine, the incidence of cerebral infarction was 22% compared with 34% in patients who received placebo, a statistically significant decrease. In addition, the rate of poor outcome was significantly reduced from 33% in the placebo group to 20% in the treatment group.(194) However, angiographic vasospasm was not reduced (195).
Other calcium channel blockers as nicardipine and diltiazem beneficial effect is inconclusive, in improving outcomes after SAH. Therefore, the exact underlying mechanism by which nimodipine is beneficial remains unknown. (196)
- Prophylactic transluminal balloon angioplasty:
A prophylactic transluminal balloon angioplasty was tested in a multi-center randomized trial. (197) No benefit was observed in the procedure, and it was responsible for 3 deaths (4%) from vessel rupture, an incidence greater than the 1.1% reported in the literature. (198)
- Clot clearance:
The process of Clearance of subarachnoid clots is speeded by the use of intracisternal thrombolysis with either recombinant tissue plasminogen activator or urokinase and there is evidence that this is associated with prevention of vasospasm and less cerebral ischemia. (199)
- Intrathecal vasodilators:
It was observed in a single-center study, nonrandomized cohort study that a Prolonged-release nicardipine implants placed in the subarachnoid space of patients with thick clots and undergoing surgical clipping results to be significantly preventable of vasospasm and ischemia. (200) Similar results were observed in a previous study with papaverine pellets being used. (201) However hopeful, this nicardipine therapy is not clear how it can be used in patients undergone endovascular clipping of aSAH.
- STATINS:
Statins, or 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, shown to be promising in preventing vasospasm. It increases the NO biosynthesis and metabolism which is the proposed mechanism of neuroprotection in vasospasm, as it leads to dilation of cerebral vessels and improved CBF. (202-204)
It was observed that although the delayed ischemic deficits (DID) were less common in the statin-treated patients, there was no significant reduction in poor neurological outcome in meta-analysis of six randomized controlled statin trials. (205) Failure in detection of any benefit in the use of simvastatin on the short or long term, when is given 40mg per day, started within 96 hours of the accident for up to 3 weeks was observed in the international, randomized, and double-blind trial STASH (simvastatin aneurysmal Subarachnoid Hemorrhage) which gathered 803 patients up till 2013. (206) Till now, patients with SAH appeared to not benefit from the addition of statins during the acute stage.
Treatment:
As discussed above, preventative methods had a limited impact established so more aggressive interventions are often implemented. The point for instituting these interventions differs widely across centers. Some intervene when there is a rising TCD velocities; others intervene in the setting of angiographic vasospasm in asymptomatic patients, while some require a neurological deterioration before starting aggressive measures. The perfect therapeutic combination is when CBP is improved, reverse or attenuate DID and low adverse events. Medical and endovascular approaches are being used in combination to reach the ideal management.
- Medical therapy:
In 1951, it was noted by denny-brown that patients with vasospasm who had concomitant hypotension often developed a worsening neurologic examination. (207-208) In 1982, Wood proposed a hematocrit goal of 30 would be ideal for oxygen transport and improved perfusion. (209) After this, the “Triple-H Therapy”: hypervolemia, hypertension, and hemodilution was proposed. Triple-H therapy theory is based on that the expanded intravascular volume and reduction in blood viscosity would be beneficial in enhancing cerebral perfusion in the setting of vasospasm. (195) Deterioration reversal was observed in in 47 of 58 patients with cerebral vasospasm when given the triple-H therapy. (210) However, no evidence has been appeared indicating that lower than normal packed red blood cell volume is beneficial in stroke as no randomized trials have been performed about it. (211) Hypertension induction is more beneficial than aggressive hypervolemia in improving cerebral oxygenation in patients with vasospasm and has fewer complications. (212) As a matter of fact, when a symptomatic vasospasm is diagnosed or strongly suspected, triple-H therapy can commence by additional volume expansion with an isotonic crystalloid infusion, which elevated CBP in vasospastic territories without a significant change in cardiac indices in euvolemic patients. (213) The risk of hemorrhage from unsecured, unruptured aneurysms in the acute and short-term natural history is not increased by hypertension and hypervolemia. (214) Triple-H therapy important risks are cardiac failure and infarction, pulmonary edema, the complications associated with either central venous catheter or PAC placement, and possibly cerebral edema and raised ICP. (215-217)
- endovascular therapy:
If goals of triple-H therapy are not easily met in a patient with persistent signs of cerebral ischemia and the neurological signs do not fade within several hours of hypertension induced therapy toward the target range, or if a patient has poor cardiopulmonary status, it is accepted to proceed directly to endovascular treatment of vasospasm. The most effective way to prevent cerebral infarction and improve neurologic function in medically refractory patients is to start aggressive treatment in less than 2 hours. (218) Delayed treatment (>2 h), however, may still be useful for a significant number of patients and should be considered even at 12–24 h period after symptom onset (219-220). Standard endovascular therapy includes transluminal balloon angioplasty and intra-arterial vasodilators infusion
- transluminal balloon angioplasty:
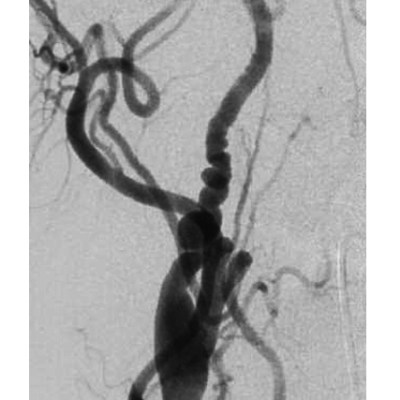
SAH induced cerebral vasospasm therapy by balloon angioplasty was firstly introduced by Zubkov and colleagues in 1984. (221) It was noted that mechanical angioplasty efficacy was nearly 100% with a complication rate of 1%. (222) In contrast to previous series, in which the efficacy ranged from 11% to 93% and a complication rate of 5% including vessel rupture in 1.1%. (223) Results of dilation in Canine models of vasospasm have shown a lasting effect for up to 3 weeks.(224-225) This is reflected on the clinical experience as balloon angioplasty is typically effective for the remainder of the vasospasm episode and patients rarely require another balloon dilation.(226-228) However, it is only effective on the artery where the balloon is inflated, and it is possible to develop symptomatic vasospasm later in an adjacent arterial segment usually distally located. Moreover, reintervention can occasionally be necessary by balloon dilation of the same vasospastic segment but it is very uncommon. (229) However, a repeated treatment is usually required for an adjacent arterial segment which is morE common. (230-231) Although there is a wide consensus that bigger vessels 2–3 mm in diameter, including intradural internal carotid artery, M1 segment of the middle cerebral artery, A1 segment of the anterior cerebral artery, V4 segment of the vertebral artery, basilar artery, and P1 segment of the posterior cerebral artery, may be considered for angioplasty treatment, (232) there is a recent report which performed 175 safe angioplasties in distal vessels without procedure-related symptomatic complications.(230)
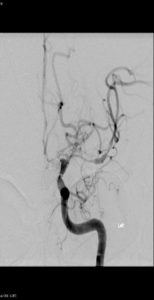
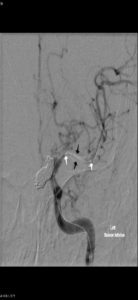

Figure 8: Balloon angioplasty for cerebral vasospasm, Case courtesy of Prof Alan Coulthard, The pre-treatment image shows vasospasm involving the left M1 and A1 segments.An endovascular balloon catheter has been passed through a guiding catheter in the left ICA and directed into the left M1 segment over a micro guide wire. The two markers indicate the proximal and distal extent of the balloon (white arrows). The balloon has been inflated in the middle cerebral artery (black arrows)After angioplasty the left MCA is of normal caliber. Left hemisphere perfusion is significantly improved.(233)
- Intra-arterial vasodilators infusion agents:
–nicardipine: it is a CCB, which is reported to have improvements in TCD velocities, stable to marginal improvements in ICPS and clinical improvements in majority of patients given nicardipine. (234-235) It mainly reverses angiographic vasospasm in treated vessels, with no sustained effect on intracranial pressure or cardiovascular function. (236)
-verapamil: A CCB, it is observed in severely spastic vessels, intraarterial administration of verapamil can results in up to 53% increase in vessel diameter (44%+- 9%). (237) It is reported to reduce angiographic vasospasm and improve the clinical picture in one third of cases without significant adverse events. (237) However, precautions should be taken into account with verapamil use as it is a negative inotrope and may cause heart block and it is known to cause seizures. (238-239)
-Nimodipine: it was discussed above as a preventive method but as an intraarterial agent, it has similar favorable good results as verapamil and nicardipine in two small retrospective series. (240-241)
-Fasudil: it is CCB which primarily works through the inhibition of the Rho-kinase signaling pathway, thus dilating blood vessels through the activation of myosin phosphatase. (195, 242-243) Angiographic improvement in all cases and clinical improvement in 44% of cases was reported on 23 consecutive patients. There was a statistically significant decline in MAP and increase in ICP. (244)
-milrinone: it is a selective phosphodiesterase III inhibitor affecting cyclic adenosine monophosphate pathways and has inotropic and vasodilatory capabilities. (245) Many scattered reports of the potential beneficial effects on the clinical and experimental vasospasm were found when milrinone is administered intravenously, Intraarterially or into subarachnoid cisterns. (246-249) Milrinone has been used to reverse CV successfully in patients with confirmed DCI.(250-254) Although a recent European survey especially in France showed milrinone potential to become the second most common drug used to treat DCI, (255) no randomized controlled trial was done searching for the neurological outcomes. Fifteen full text journal articles were identified using milrinone arterially or venously to treat patients with confirmed DCI in a recent systematic analysis. (256)
Prognosis:
The prognosis of patients who had cerebral vasospasm and DCI after aSAH is strongly proportional to the severity of SAH and the clinical condition of patient once the accident had occurred. The risks of severe vasospasm is strongly related to those factors. It is necessary to consider angiographic vasospasm is not always correlated to clinical deterioration of the patient. In conclusion, patients suffering from vasospasm are at high risk of cerebral ischemia thus poor neurological outcome. (257)
On the horizon “future directions to cerebral vasospasm”
Unfortunately to date, the present literature provides few definitive answers on the strategies of decreasing the impact of the vasospasm following SAH. There are many factors contributing to the difficulty in defining the treatment and preventive methods of vasospasm as the incompletely understood underlying mechanism of vasospasm, complexity of SAH make it hard. Multiple neuroprotective approaches as well as the utilization of multimodal treatment regimens are being constructed and hold promising results in treatment or preventing vasospasm. (258) Some of the future direction will be discussed here as :
-Enoxaparin:
A randomized clinical trial was done in SAH to study this drug which is a low molecular weight heparin. (259) The results showed decline in the incidence of DID and infarcts but the admission characteristics of the two groups were not well balanced.
-Nitric oxide donors:
It is a free radical gas formed from the substrate L-arginine. In 1987, it was discovered and appeared to play an important role in controlling cerebral vasomotor tone. (260) The release of NO is considered an important regulator of resting CBF as inhibiting NO release constrict Cerebral arteries and decrease CBF.(261-263) Sodium nitroprusside a NO donor given intraventricularly to patients with medically refractory vasospasm had diverse impacts on CBF and a high rate of adverse events (264) In a study where intrathecal nitroprusside was administered it resulted in clinical and angiographic improvement of vasospasm and excellent outcome without systemic or neurological complications in three patients(265). CTP scan was done and it had resulted in increased CBF in the nitroglycerin group (266). At present, large randomized and controlled trials of NO donors in SAH are in the planning stage.
-Endothelin-1 antagonists:
Clazosentan, an ETA antagonist showed decline in the incidence and severity of angiographic vasospasm in a study of phase IIa trial (267). In addition, Side effects were close to placebo. furthermore, ETA/B antagonist, TAK-044, was also studied in a phase II trial (268). This drug was acceptably tolerated and it had resulted in Delayed ischemic deficits which occurred in 29.5% of patients taking active treatment and 36.6% of patients on placebo (risk reduction by 0.8). clazosentan was tested very recently in a controlled clinical trial registering 413 patients with SAH (269). It had resulted in considerable reduction in Moderate to severe angiographic spasm, although there was no effect on outcome.






genius
Thank you for these value informations.
Every thing was nice.
Good arrangement and flow of the thoughts.
Thank you
Thank you for these value informations.
Every thing was nice.
Good arrangement and flow of the thoughts.
Thank you
Every thing was nice.
Arrangement and flow of thoughts is great.
Thank you for these value informations.
Thank you.