Author: Abdalrhman Albassomi
Editors: Rana Aqeel, Sherine Asha.
Reviewers: Adam Abdulla, Ethar Hazaimeh
Overview
Vein of Galen malformation (VOGM) is a rare congenital anomaly of the cerebral vasculature. It is characterized by a direct connection between the arteries and veins of the brain, bypassing the capillary network. This connection forms a high-flow shunt that can overload the heart, leading to congestive heart failure (CHF), among other complications. Although this malformation constitutes less than 1% of all intracranial arteriovenous malformations, it represents 30% of all pediatric intracranial vascular malformations [1].
Etiology and Pathogenesis
The exact etiology of VOGMs is unknown, but they are thought to be caused by a combination of genetic and environmental factors. Although rare, some cases have been associated with genetic syndromes. These include RASA1 mutations, linked to capillary malformation-arteriovenous malformation (CM-AVM) syndrome, and ACVRL1 mutations, associated with hereditary hemorrhagic telangiectasia (HHT) [2,3].
Early in fetal development, a temporary embryonic vein called the median prosencephalic vein of Markowski drains the choroid plexus into the falcine sinus, a dorsal interhemispheric venous network. During weeks 6-11 of gestation, the internal cerebral veins and Rosenthal basal veins, located behind the pineal gland, fuse to form the vein of Galen (VOG), which assumes the drainage function of the median prosencephalic vein. As the basal ganglia grow and the VOG develops, the median prosencephalic vein normally regresses by the 11th week of gestation. If this regression fails, an arteriovenous fistula can develop between the persistent median prosencephalic vein and the embryonic choroidal arteries, causing progressive dilation and preventing normal involution, as shown in Figure 1 and Video 1 [4]. This abnormal connection, known as a VOGM, is supplied by multiple deep cerebral arteries, most commonly the posterior choroidal, anterior choroidal, and thalamoperforating arteries [5].
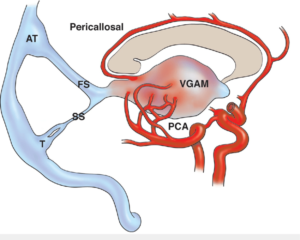
Figure 1. Illustration of VOGM and its feeding arteries [6].
Diagnosis
The diagnosis of VOGMs is typically postnatal, but it can be detected antenatally in approximately 29% of cases. On antenatal ultrasound, VOGMs are generally observed from 25 weeks of gestation and may appear as cystic midline brain lesions, as demonstrated in the ultrasound image, Figure 2. Color Doppler imaging can then reveal abnormal blood flow, a finding sometimes referred to as the ‘comet tail’ sign, as shown in Figure 3. Antenatal magnetic resonance imaging (MRI) can also be used to confirm the diagnosis. It allows evaluation of any brain injury and helps with planning delivery in a facility with the necessary resources and expertise [7].
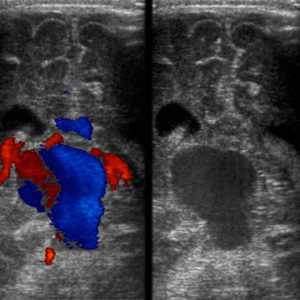
Figure 2. Transcranial ultrasound of the brain. Left: Doppler examination demonstrating abnormal flow within the vein of Galen with aneurysmal dilation. Right: Grey-scale image at the same level showing the aneurysmal dilation of the VOG [8].
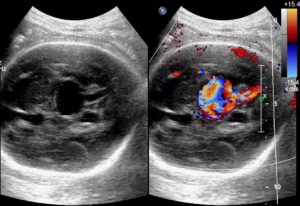
Figure 3. Antenatal ultrasound demonstrating the ‘comet tail’ sign [9].
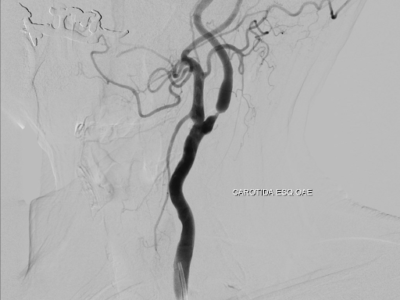
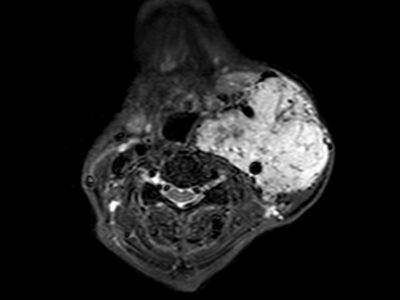
In infants and children, the malformation more commonly presents with neurological symptoms, such as hydrocephalus, macrocephaly, and seizures. VOGM can rarely present in adults, and it typically presents with headache, vomiting, seizures, or subarachnoid hemorrhage [1,11]. Computed tomography (CT) and MRI should be used for comprehensive assessment of the arteriovenous shunt and associated brain changes. CT angiography provides rapid and detailed vascular information, often superior to ultrasound or MRI in depicting both arterial feeders and venous drainage, as demonstrated in the arterial phase images, Figures 4 and 5. MRI is the modality of choice for evaluating the ventricular system and cerebral parenchymal damage, which is important for treatment planning [1].
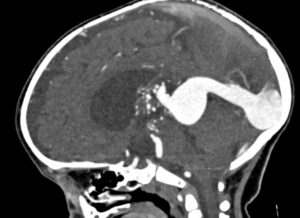
Figure 4. Sagittal view of cerebral CT angiography of VOGM during the arterial phase [12].
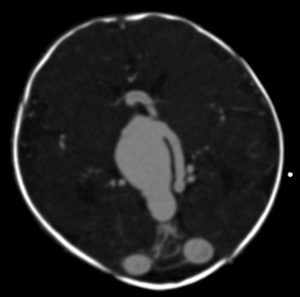
Figure 5. Axial view of cerebral CT angiography of VOGM during the arterial phase [13].
VOGM has two main classification systems: Lasjaunias and Yasargil classifications. Lasjaunias classification mainly depends on the angioarchitecture of the fistulae, while Yasargil classification categorizes them based on the relationship between the malformation and the VOG [1,14].
- Lasjaunias Classification
Depending on the angioarchitecture of the fistula, VOGMs can be divided into choroidal and mural types, as shown in Figure 6. The mural type consists of high-flow shunts that end up within the aneurysmal wall of the median prosencephalic vein and are supplied by the collicular and posterior choroidal arteries. The choroidal type requires the formation of a sizable arterial network between the venous aneurysm and the arterial feeder, which is fed by the choroidal, subfornical, pericallosal, and/or thalamoperforator arteries [1]. The mural form is generally associated with milder clinical symptoms and typically presents in infants and children compared to the choroidal form, which causes more severe symptoms and presents early in neonates [15].
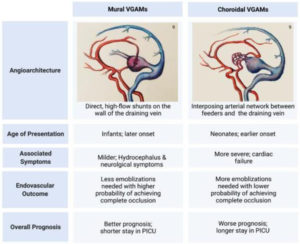
Figure 6. Lasjaunias Classification of VOGMs [16].
- Yasargil classification
According to the relationship of the malformation to the VOG, the Yasargil classification categorizes VOGMs into 4 types as shown in Table 1. Types I to III are referred to as true VOGMs as they form a direct fistulous connection with the VOG without a nidus, while type IV is a true parenchymal AVM that drains into the vein of Galen via the internal cerebral vein, median atrial vein, or basilar veins [14].

Table 1. Yasargil Classification of VOGMs [14].
VOGMs can lead to a number of complications, ranging from life-threatening to long-term neurodevelopmental deficits. The high-output state caused by the anomalous shunt may result in severe CHF. Impaired venous drainage can contribute to devastating neurological sequelae, such as hydrocephalus, hemorrhagic stroke, and seizures. Survivors are at risk of developmental delays, cognitive impairment, and functional deficits such as speech impairment, visual and auditory disturbances. In severe cases, these complications may culminate in death [1,16].
Management
The primary goal of VOGM’s treatment is to reduce shunt flow through the malformation, thereby alleviating the high-output cardiac burden and minimizing the risk of venous congestion-related complications. The most common treatment options are: Endovascular embolization, stereotactic radiosurgery (SRS), and surgical management, as shown in Figure 8 [16].
- Endovascular Embolization
The management of VOGMs has witnessed a significant advancement since the development of endovascular embolization in the early 1980s. The mainstay of management is transfemoral, transarterial embolization. Pre-therapeutic brain MRI and MRA are performed under general anesthesia to guide treatment planning. During embolization, a flow-guided microcatheter is used to deliver high-concentration N-butyl cyanoacrylate (n-BCA). To balance the effectiveness of embolization against the risk of cerebral impairment, the procedure is typically performed in infants between 4 and 6 months of age [16,17]. Endovascular embolization remains the first-line treatment, as it offers acceptable mortality and complication rates, along with favorable clinical outcomes [18].
- Stereotactic Radiosurgery (SRS)
SRS is a technique that utilizes focused beams of radiation to damage the anomalous blood vessels, thus reducing blood flow through the malformation. Several technologies can deliver SRS, the most established being the Gamma Knife, which uses multiple converging cobalt-60 beams to precisely target the lesion [19,20]. SRS is typically considered a second-line therapy after unsuccessful embolization. While it can reduce the size of the malformation and its arterial feeders, a major limitation is that it does not provide immediate hemodynamic relief, since vascular closure occurs gradually through radiation-induced endothelial changes [20].
- Surgery
Surgery is a more invasive procedure than endovascular embolization and SRS, and carries a higher risk of complications. Thereby, it is typically reserved for VOGMs that are not amenable to endovascular embolization or SRS. It can also be utilized in the treatment of VOGM’s complications, such as hydrocephalus and the evacuation of intracranial hematomas [1,16].
- Other Treatment Options
Other treatment options may be used for VOGM, depending on the individual patient’s needs. Medical management is typically given with the aim of stabilizing cardiopulmonary and neurological symptoms before endovascular intervention [16]. Patients with VOGM may also benefit from physical therapy to improve motor skills and from speech therapy to enhance language abilities [21].
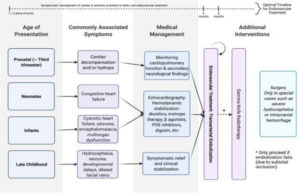
Figure 8. Schematic Overview of Management Strategies in VOGM [16].
Without proper treatment, VOGMs typically have a fatal prognosis, with a mortality rate of approximately 80-100% of cases [10]. The outcome is strongly influenced by the cardiac and neurological status at presentation and the patient’s age, with neonates presenting with severe high-output cardiac failure or hydrocephalus at the greatest risk. Advances in endovascular management and supportive care have significantly improved survival and functional outcomes [16].
References