Article Title: Fear and Reward Circuitry: Insights from Neuroscience
Author: Amani Alfdool
Editor: Aseel Qandil
Reviewer: Ethar Hazaimeh
Keywords: Limbic system, Amygdala, Fear, Reward, Emotions
Abbreviations: PFC (Prefrontal Cortex), VTA (Ventral Tegmental Area), BNST (Bed Nucleus of the Stria Terminalis), DBS (Deep Brain Stimulation), SSRIs (Selective Serotonin Reuptake Inhibitors), CeA (Central Nucleus of the Amygdala), BLA (Basolateral Complex of the Amygdala), OFC (Orbitofrontal Cortex), CRF (Corticotropin-Releasing Factor), fMRI (Functional Magnetic Resonance Imaging), GABA (Gamma-Aminobutyric Acid), PTSD (Post Traumatic Stress Disorder).
Abstract
After a long period of neglect, neuroscience has again recognized emotion as a crucial area of study. This renewed attention highlights the limbic system’s role in building our emotions and influencing decision-making, with reward and fear circuits driving survival behaviors. Understanding these systems has broadened our theoretical knowledge about behavior and emotional regulation, as well as our development of innovative therapies, including exposure therapy and deep brain stimulation, for conditions such as anxiety, addiction, and PTSD.
This article will provide a brief overview of the anatomy and function of fear and reward circuits, their interactions, and the therapeutic implications.
Introduction
The limbic system is the brain’s emotional control center; it shapes emotional experiences and decision-making by regulating key emotions such as fear, survival, and reward (1). Fear circuits anchored by the amygdala and hippocampus allow quick threat detection and defensive responses, while reward circuits, driven by dopamine signaling in the nucleus accumbens, promote life-sustaining behaviors (2). Reward circuits encourage positive behaviors, while fear circuits defend against threats (3). The way these circuits interact can affect mental health. Psychiatric disorders like anxiety and addiction may be exacerbated by dysfunctions in these systems (4).
Recent studies have shed light on how these circuits function and their potential for new therapeutic options. For example, studies indicate that fear extinction, essential for PTSD treatment, is facilitated by the interactions between the amygdala and prefrontal cortex (PFC) (5). Additionally, reward circuits use subtle dopamine signals to encode prediction errors, which enhance adaptive learning (6). Psychiatric conditions such as anxiety, addiction, and PTSD are caused by impairment in these systems (5). Deep brain stimulation (DBS) and optogenetics are two recent neurotechnological advancements that present promising therapeutic options (1).
This article offers a comprehensive overview of the limbic system’s anatomy, the neural circuits involved in fear and reward, their clinical significance, and emerging therapeutic approaches. Additionally, Insights from animal models and human research were analyzed to show how these systems influence behavior and resilience (7).
The limbic system’s anatomy
The limbic system consists of interconnected structures that regulate motivation, emotions, and memory (8). The amygdala in the medial temporal lobe processes fear and aggression by evaluating sensory input for potential threats (2). It is divided into several nuclei, including the central nucleus (CeA), which regulates autonomic responses, and the basolateral complex (BLA), which plays a key role in fear learning (9).
The hippocampus, located next to the amygdala, helps contextualize fear memories by integrating temporal and spatial cues- time and place, respectively- to prevent fear from being overly generalized. (1). Research has shown that Hippocampal lesions impair the ability to differentiate between dangerous and safe environments(10).
The prefrontal cortex (PFC) helps to regulate excessive fear by exerting top-down control over the amygdala through its connection with the BLA (11). This regulatory function is crucial for extinction learning, where repeated exposure to non-threatening stimuli reduces the fear responses (5).
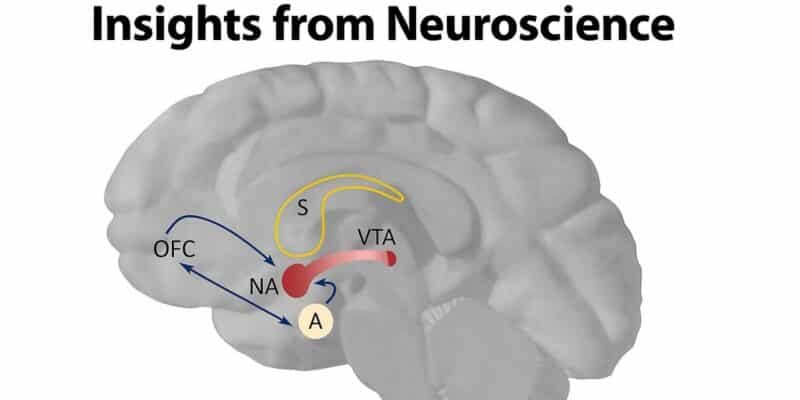
The nucleus accumbens, a key part of the ventral striatum, drives reward-seeking behaviors by releasing dopamine from the ventral tegmental area (VTA) (6). When behaviors lead to favorable results, this triggers the dopamine release in the nucleus accumbens, encouraging the repetition of those actions associated with rewards by signaling whether the rewards were better or worse than expected, which indicates errors in reward anticipation”(12).
The bed nucleus of the stria terminalis (BNST) is another structure that integrates reward and fear signals to regulate emotional balance and stress reactions (13). When BNST activity is impaired, it is linked to anxiety and addiction, highlighting its crucial role in maintaining homeostasis (14).
Fear Circuitry
Fear processing begins when the thalamus sends sensory signals to the amygdala through the “low road” pathway, enabling rapid threat detection before cortical processing occurs (15). This pathway results in an immediate defensive response, such as freezing or escaping. In contrast, the “high road” (thalamus → sensory cortex → amygdala) provides a more detailed analysis of the situation (9). The amygdala controls these reactions through hypothalamus activation, which initiates fight-or-flight responses, and the brainstem, which mediates autonomic changes (16).
The hippocampus plays a dual role in fear processing by contextualizing fear memories. In fear conditioning studies, for example, rodents learn to link an auditory tone (neutral stimulus) with an electric shock (unpleasant stimulus), which depends on the interactions between the hippocampus and amygdala(1).
In individuals with PTSD, brain imaging studies show amygdala hyperactivity when exposed to trauma-related reminders (17).
During fear extinction, GABAergic interneurons facilitate the prefrontal cortex (PFC) inhibition of amygdala activity (5). When the PFC’s function is impaired, as seen in anxiety disorders, extinction fails to occur, leading to persistent fear (18). Therapies such as exposure therapy aim to strengthen connectivity between the PFC and amygdala, helping to alleviate symptoms (19).
Reward Circuitry
Reward is essential for reinforcing incentive-based learning, shaping appropriate responses to stimuli, and supporting goal-directed behaviors. Dopamine-driven circuits play a key role in enhancing survival behaviors by reinforcing adaptive responses(6).
Dopamine neurons in the VTA monitor prediction errors that drive learning by transmitting signals to the nucleus accumbens (12). Moreover, dopamine levels increase when a reward exceeds what is expected (positive prediction error) and decrease when it is less than expected (negative prediction error) (20). This process influences adaptive behaviors, such as forming social connections (21).
When dopamine levels increase artificially, the prefrontal cortex’s ability to regulate impulsive behaviors weakens (22). Studies on animals show that frequent cocaine exposure reduces gray matter in the prefrontal cortex, leading to impaired decision-making (23).
Similarly, different neurons in the ventral tegmental area (VTA) play distinct roles in reward processing. For example, lateral hypothalamic neurons are involved in reward consumption, while VTA neurons contribute to reward anticipation (24).
The orbitofrontal cortex (OFC) plays a crucial role in assessing reward value and adjusting decision-making based on new information (25). In cases of addiction, OFC dysfunction contributes to compulsive drug-seeking behavior despite negative consequences (22).
Interactions Between Fear and Reward Circuits
Fear and reward circuits are balanced by weighing risk and benefit. The amygdala, which drives avoidance, and the nucleus accumbens, which encourages approach, work in opposition to regulate behavior in approach-avoidance situations. Additionally, the PFC plays a role in decision-making during these conflicts(26).
For example, depending on hunger levels, a mouse might avoid a predator due to fear but approach food due to the desire for reward (9).
By integrating these signals, the bed nucleus of the stria terminalis (BNST) regulates stress responses through the release of corticotropin-releasing factor (CRF) (13). In anxiety, heightened BNST activity amplifies fear responses, while in addiction, it diminishes reward sensitivity (14). Studies indicate that drug cravings are exacerbated by stress-induced CRF release in the BNST (27).
Counterconditioning is a therapeutic method that uses reward circuits to suppress fear. For example, in phobia treatment, pairing a feared stimulus with a rewarding experience can reduce amygdala activity, helping to alleviate fear (10).
Clinical Implications and Therapeutic Advances
Research into reward and fear circuits has led to various treatments. Exposure therapy helps patients overcome fear by repeatedly exposing them to their fear within controlled and safe settings, strengthening neural connections between the prefrontal cortex (PFC) and the amygdala (19). The effectiveness of cognitive-behavioral therapy (CBT) in emotional regulation comes from its ability to activate the PFC, which reduces amygdala overactivity (28).
Deep brain stimulation (DBS) is used for treatment-resistant depression and obsessive-compulsive disorder (OCD), targeting regions like the amygdala or nucleus accumbens. A study found that 80% of patients experienced a 50% reduction in depressive symptoms when using closed-loop DBS, which adjusts stimulation based on neural activity (29). Additionally, techniques like optogenetics allow precise control of neuronal groups, improving our understanding of circuit dysfunction in conditions such as autism and schizophrenia (29).
The administration of medications such as serotonin reuptake inhibitors (SSRIs), can improve PFC’s control over fear and reward circuits. SSRIs increase serotonin levels in the PFC, which improves emotional regulation in patients with anxiety (30)(31).
Conclusion
The reward and fear circuits in the limbic system play a critical role in the survival and overall mental health of an individual. When their function is impaired, this can lead to psychiatric disorders. While new technologies offer hope for developing more personalized treatments, maintaining balance in these systems is essential to promote overall resilience and well-being.
References
[Read more]
1. Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci [Internet]. 2015;16(6):317–31. Available from: http://dx.doi.org/10.1038/nrn3945
2. Ledoux JE. The amygdala, fear, and memory. Annu Rev Neurosci [Internet]. 2000;23:155–84. Available from: 10.1146/annurev.neuro.23.1.155.
3. Berridge KC, Kringelbach ML, Arbor A, Hospital W. Pleasure Systems of the Brain. Neuron. 2016;86(3):646–64.
4. Zhao L. Gradient estimates for a simple parabolic lichnerowicz equation. Osaka J Math. 2014;51(1):245–56.
5. Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36(4):567–84.
6. Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology [Internet]. 2010;35(1):4–26. Available from: http://dx.doi.org/10.1038/npp.2009.129
7. MICHA R. 乳鼠心肌提取 HHS Public Access. Physiol Behav. 2017;176(1):100–106.
8. Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci. 2014;8(APR):1–6.
9. Janak PH, Tye KM, Sciences B, Sciences C. From circuits to behavior in the amygdala : Nature : Nature Publishing Group. NatureCom [Internet]. 2015;517(7534):284–92. Available from: http://www.nature.com/nature/journal/v517/n7534/pdf/nature14188.pdf%5Cnpapers3://publication/uuid/F2672197-C748-4CA9-9E69-C39B97C33FEE
10. Ressler KJ. 基因的改变NIH Public Access. Biol Psychiatry. 2010;67(12):1117–9.
11. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–73.
12. Deisseroth K. Optogenetics. 2011;8(1):26–9.
13. Widge AS, Zorowitz S, Basu I, Paulk AC, Cash SS, Eskandar EN, et al. Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nat Commun [Internet]. 2019;10(1):1–11. Available from: http://dx.doi.org/10.1038/s41467-019-09557-4
14. Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–34.
15. Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–87.
16. Maren S. N Eurobiology of P Avlovian. Neuroscience. 2001;897–931.
17. Gozzi A, Schwarz AJ. Large-scale functional connectivity networks in the rodent brain. Neuroimage. 2016;127:496–509.
18. Gloria Kang GJ, Ewing-Nelson SR, Mackey L, Schlitt JT, Marathe A, Abbas KM SS. 乳鼠心肌提取 HHS Public Access. Physiol Behav. 2018;176(1):139–48.
19. Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–51.
20. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology [Internet]. 2010;35(1):169–91. Available from: http://dx.doi.org/10.1038/npp.2009.83
21. Brokowski C AM. 乳鼠心肌提取 HHS Public Access. Physiol Behav. 2019;176(5):139–48.
22. Wise RA. Dopamine, learning, and motivation. Nat Rev Neurosci. 2004;5(6):483–94.
23. Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53.
24. Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science (80- ). 2019;363(6424):276–81.
25. Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci. 2011;34:289–307.
26. Scangos KW, Khambhati AN, Daly PM, Makhoul GS, Sugrue LP, Zamanian H, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med [Internet]. 2021;27(10):1696–700. Available from: http://dx.doi.org/10.1038/s41591-021-01480-w
27. Roth BL. DREADDs for Neuroscientists. Neuron [Internet]. 2016;89(4):683–94. Available from: http://dx.doi.org/10.1016/j.neuron.2016.01.040
28. Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–9.
29. Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci [Internet]. 2012;13(4):251–66. Available from: http://dx.doi.org/10.1038/nrn3171
30. Volkow ND, Wise RA, Baler R. The dopamine motive system: Implications for drug and food addiction. Nat Rev Neurosci. 2017;18(12):741–52.
31. Howell LL, Cunningham KA. Serotonin 5-ht2 receptor interactions with dopamine function: Implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67(1):176–97.
[/read]