Article topic: Mesial temporal sclerosis
Author: Abdullah Alshloul
Editor: Hamzeh Aljarrah
Reviewer: Rahmeh Adel
keywords: Mesial temporal sclerosis, Hippocampal Sclerosis, Classification, Diagnosis, treatment, complications
Abbreviations:
MTS= Mesial temporal sclerosis, HS= hippocampal sclerosis, CA=cornu ammonia, AD=Alzheimer Disease, SNP= single nucleotide polymorphism, TLE= Temporal Lobe Epilepsy.
Abstract
Mesial temporal lobe sclerosis is hippocampal sclerosis with more extensive sclerosis of adjacent structures in the medial temporal lobe, including the amygdala and parahippocampal gyrus.2 MTS can be classified into three types. 1 It may occur alone or with a combination of other pathologic disorders.19
Some risk factors that can lead to MTS may be genetic, environmental, and a malformation in part of the medial temporal lobe. Temporal lobe seizure is the most common clinical feature of MTS.36
There are many methods for diagnosing MTS by scans, including CT, and MRI, and all show atrophy of the hippocampus, with an increase in the intensity of the signal on T2-weighted sequences.71 Treatment of MTS either pharmacologically or surgically.
Overview
The mesial temporal lobe comprises the parahippocampal gyrus, uncus, hippocampus, fimbria, dentate gyrus, and amygdala91.
Mesial temporal sclerosis (MTS), also called hippocampal sclerosis (HS), is neuronal loss and gliosis involving the hippocampus and often the amygdala, uncus, and parahippocampal gyrus.1
The most common cause of medically intractable partial complex epilepsy in adults is (MTS). It’s a common pathology encountered in mesial temporal lobe epilepsy (MTLE), other epilepsy syndromes, and in both surgical and post-mortem practice.1 MTS classification segregates into typical (type 1) and atypical (type 2 and 3) groups, depending on the histological patterns of subfield neuronal loss and gliosis.1
| Pathology type | Definition |
| Amygdala gliosis/sclerosis | Gliosis ± neuronal loss in the amygdala (lateral and basal nuclear groups) |
| Parahippocampal gyrus/entorhinal cortex | Laminar neuronal loss and gliosis |
| Temporal neocortical sclerosis | Neuronal loss was reported in 11% of HS/TLE patients in layer II/III. |
| Widespread cortical atrophy | Subtle volume loss/cortical thinning was reported in both ipsilateral (and bilaterally) including temporal, frontal, and parietal cortical regions in MRI and PM studies |
Table 1. Summary of the pathologic forms of MTS 2
Hippocampal Anatomy
The hippocampus, located in the medial temporal lobe, consists of the dentate gyrus, cornu ammonis (CA1-4), and the subiculum.25,2
The laminar structure of the hippocampus from the outside comprises the alveus (outgoing axons), stratum oriens (crossing axons and interneurons), stratum pyramidale (the main cellular elements, the pyramidal cells), stratum radiatum (apical dendrites of pyramidal cells), and stratum lacunose/molecular (axon bundles, dendrites, and interneurons) (Figure 1).2
Several schemes have subdivided the hippocampal subfields, such as the system of Lorente de No (Figure 1 & 2). The pyramidal cell layers have four subfields (CA1–4); CA1 is the largest region and the broadest part of the pyramidal cell layer and is in continuity with the subiculum; CA2 is narrower and contains densely packed neurons. CA3 forms the hippocampus’s curve. CA4 is located within the dentate gyrus’s concavity and is made up of scattered ovoid cells, also known as the endplate or end blade.2
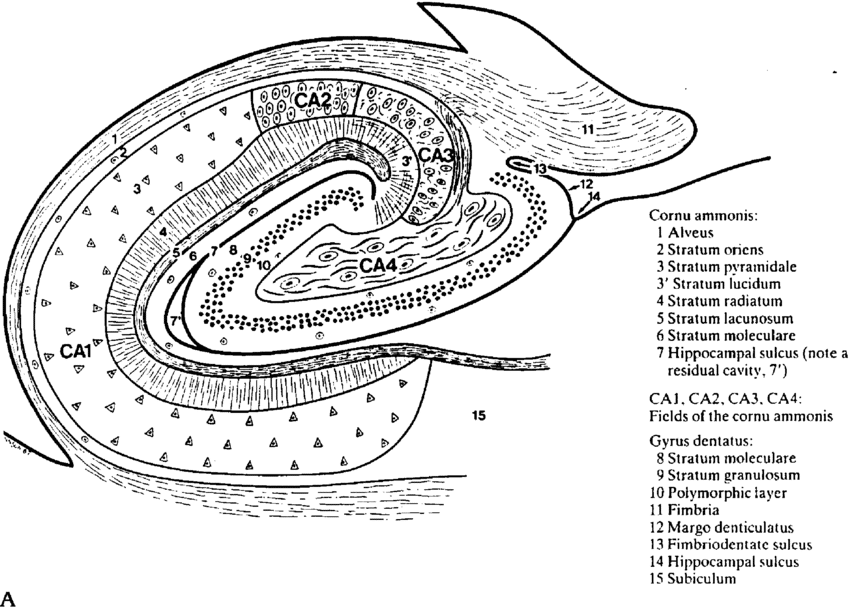
Figure 1: Hippocampal morphology and cytoarchitecture. Diagram of a transverse section of the hippocampal body.90
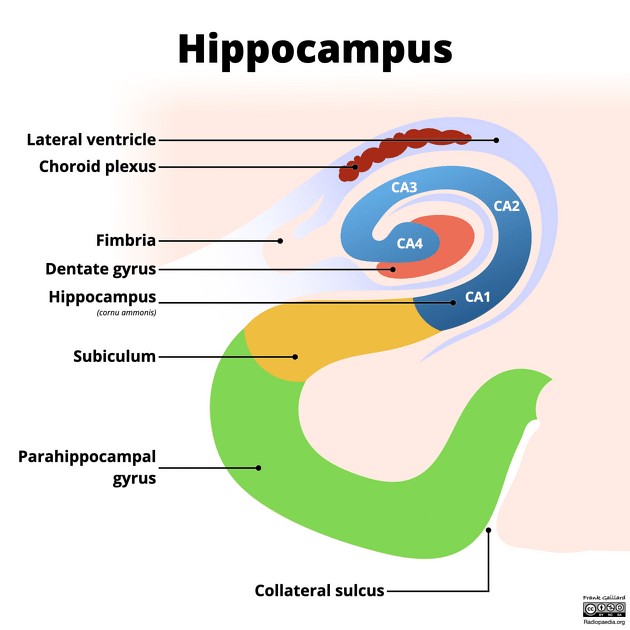
Figure 2: Hippocampus structure.89
MTS Classification
There are many attempts to make a specific classification for MTS patterns and correlate subtypes with postsurgical results. There are five distinct pathology patterns of MTS: (no MTS) = “no hippocampal cell loss”; (MTS type 1a) =” classic hippocampal sclerosis”; (MTS type 1b) = “severe hippocampal sclerosis”; (MTS type 2) = “CA1 sclerosis”; and (MTS type 3) = “end folium sclerosis”.23
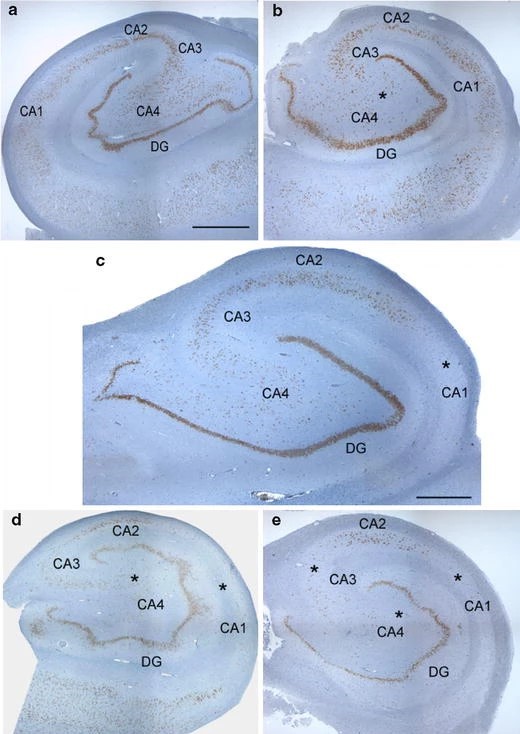
Figure 3 Classification of MTS.
Patterns of MTS. (a) No MTS, (b) MTS type 3 (end folium sclerosis), (c) MTS type 2 (CA1-sclerosis), (d) MTS type 1a (classic hippocampal sclerosis), and (e) MTS type 1b (severe hippocampal sclerosis).24
Epidemiology
Hippocampal sclerosis can occur alone or with a combination of other conditions such as Alzheimer’s disease (AD), Vascular dementia (VaD), Lewy body dementia (LBD), or argyrophilic grain disease. Prevalence equals 16% and more in patients older than 80 years of age.19
Many autopsies have shown that 5-30% of brains in the elderly age harbor HS-aging pathology.21 Advanced old age is associated with a high prevalence of aging pathology, as indicated by epidemiological data. Furthermore, this pathological condition has been found to have a strong correlation with cognitive impairment, regardless of the presence of Alzheimer’s disease pathology.22
The hippocampus is the most frequently involved region and the most vulnerable structure in patients with Temporal Lobe Epilepsy (TLE).74
Risk factors
The hippocampus is particularly susceptible to hypoxic injury.44 The association between vascular risk factors and HS has been suggested by many studies.20,45,46 Stroke and white matter disease have effects on the hippocampus and a high association between HS with arteriolosclerosis. 45,46
TAR DNA binding protein 43 (TDP-43) immunoreactivity is highly prevalent in HS patients and was found in approximately 90% of HS patients.47
The presence of the SNP in TMEM106B, GRN, and ABCC9 alleles plays a role in HS. 51 The presence of Single nucleotide polymorphisms (SNP) rs1990622 in the (TMEM106B) gene may be related to HS. Among the GRN mutation variants, the SNP rs5848 may play a role in HS.48,49
The polymorphism in the ABCC9 gene was also associated with an increased risk for HS.50 Two ABCC9 SNPs, (rs704178) and (rs704180), in linkage disequilibrium, found an odds ratio of 2.1 for HS. This gene is known as sulfonylurea receptor 2, and this drug class antagonizes the protein encoded by the ABCC9 gene. In this context, sulfonylurea exposure after age 85 was associated with an increased risk of HS. 51 Urea cycle disorder can lead to MTS which leads to increased susceptibility of patients to seizures regardless of their metabolic state.52
MTS may have developed after treatment of hematologic cancers such as leukemia or lymphoma.61
Etiology
The etiology of (MTS) is still contentious and is likely to be multifactorial. It is generally believed to be an acquired condition. 1
Initiating insult and genetic susceptibility factors
In retrospective studies of patients with hippocampal sclerosis, it is commonly observed that a noteworthy brain injury or an initial triggering event, typically experienced during early life, such as a febrile or prolonged seizure, is frequently reported. The occurrence of such events has been documented in approximately 30-50% of cases, and even up to 80% in a specific surgical series.3
The “injury” hypothesis suggests that when the hippocampus is insulted, it can lead to irreversible damage or changes. This can create a template for the development of hippocampal sclerosis after a period of latency. It seems that there is a specific age sensitivity to this injury, as a more significant loss of neurons is observed when the initial insult occurs between the ages of 4 and 7 years.4
Seizure-induced neuronal loss
Seizure-induced neuronal death is primarily caused by an excessive influx of sodium (Na+) and calcium (Ca2+) through excitotoxic glutamatergic neurotransmission. This leads to osmolytic stress, resulting in cell swelling and rupture. Additionally, it triggers the production of free radicals that damage DNA and activate proteases, leading to the breakdown of cell and organelle membranes. Ultimately, these processes culminate in necrosis.5
Inflammation
Infections have been identified as potential factors contributing to HS. While there is evidence linking it to neurocysticercosis, it is important to note that this association is more likely due to a dual pathology rather than a direct impact on the hippocampus.6
Viral encephalitis commonly affects the mesial temporal structures, leading to the development of bilateral (HS) and severe memory problems. 7
In Hippocampal Sclerosis, it is sometimes observed that there are occasional findings of neuronophagia or focal infiltrates of microglia. Studies have provided evidence of activation in both the innate and adaptive immune system in (HS), such as upregulation of IL-1β and IL-1 receptors in astrocytes, microglia, and neurons. There is also an expression of intercellular adhesion molecule 1 (ICAM-1) and kallikrein in glial cells.8,9 However, B and T cell infiltrates are usually inconspicuous and predominantly located around blood vessels.8-11
In human temporal lobe epilepsy (TLE), gene expression studies support the activation of inflammatory pathways, which is believed to contribute to disease progression.12,13
Injury
Abnormalities of the hippocampus are known to be associated with temporal lobe seizures and memory dysfunction, there is a link between head trauma and the development of a seizure focused on the temporal lobe (i.e., temporal lobe epilepsy).14
Genetic abnormalities
Temporal lobe epilepsy is commonly considered an acquired condition primarily influenced by environmental factors, with a limited genetic impact.15
Mutations in the SCN1A gene, which codes for a sodium channel subunit found in the brain, are responsible for various rare forms of epilepsy characterized by febrile seizures. This gene is a target for numerous anti-epileptic medications. 16
Maldevelopment of the hippocampus
Recent research has revealed significant discoveries regarding maldevelopment abnormalities observed in individuals with HS. These findings indicate distinct neuropathological patterns that are consistent with early onset or delayed maturation of the cytoarchitecture and molecular organization of the hippocampus in patients with temporal lobe epilepsy (TLE).17
Interestingly, minimal dysgenetic changes have been observed in neighboring structures of the temporal lobe, such as the white matter, amygdala, and entorhinal region. These observations offer valuable insights for future investigations into signaling cascades regulated during development, which may serve as crucial factors in understanding HS-associated TLE.17
Malformations
There is an association between (HS) and lesions on temporal and extratemporal areas such as cortical, vascular malformations, and small ratio tumors. 18
Pathogenesis
Excitotoxic Amino Acid Receptors
The two most prevalent amino acids in the brain are Excitatory Amino Acids (EAAs); L-glutamate and L-aspartate. Given the high concentration and extensive distribution of these (EAAs) in the central nervous system, it is not unexpected that EAAs play a significant role in normal neuronal activities and contribute to the cellular abnormalities of some pathologic states.25
Activation of some receptors in CNS such as N-methyl-D-aspartate (NMDA) may lead to neuronal cell death.26 Studies showed that activation of non-NMDA receptors is required for glutamate-induced neuronal injury, or the activation of non-NMDA inotropic receptors causes a lethal accumulation of Ca2+ to happen in the slow neurotoxicity by voltage-operated Ca2+ channels that are considered a pathway for Ca2+.27,28
Energy Metabolism Defects
Alterations in cerebral blood flow and changes in brain metabolism frequently accompany prolonged seizures. The higher metabolic needs of the seizure cause a change in cerebral blood flow as a result.
During the early stages of a seizure, blood pressure increases and blood flow rises significantly. This initial rise in blood flow has a protective effect by delivering more oxygen and glucose to the brain. However, as the seizure progresses, blood flow begins to decrease as blood pressure falls. The later reduction in blood flow limits the supply of oxygen and glucose reaching the brain.25
Ischemia, hypoxia, hypothermia, hypertension, and hypoglycemia are further systemic alterations that can occur after seizures. 32 Massive increases in arachidonic acid concentration, diacylglycerol-mediated activation of protein kinase C, calcium-mediated alterations in calmodulin kinase II, and production of free radicals have all been linked to seizure-induced systemic and metabolic abnormalities. 33 These elements might be involved in how neuronal damage occurs.
Gene Expression
The happening of seizure leads to rising levels of mRNA and proteins that are encoded by the immediate early genes c-fos, c-jun, jub-B, and (NGFI-A), The significance of expressions and function of these genes is unknown. 34,35 Many studies suggested that gene expression maybe involved in seizure-induced cell death.25
Clinical presentation
The most important feature of MTS is repeated temporal lobe seizure, which is very hard to control pharmacologically and is observed in patients with neuropsychological defects which can be shown by suitable tests.36 Also, frequent seizures happen during early age (6-10) years old, with the presence of some type of aura. The only significantly related kinds are visceral, olfactory, and uncinate. A pattern of conduct is typical of ictus, although this is not specific: Early ipsilateral manual automatism and contralateral tonic posture and Infrequent generalization. 36
Individuals with MTS have the same initial symptoms and rates of dementia development as those with Alzheimer’s disease (AD) and therefore are frequently misclassified as having AD.37 The signs and symptoms of MTS are generally the same as those seen in AD, with recent memory loss being clear. 37 Behavioral disturbances and subtle neuropsychologic deficits may differentiate patients who have MTS from those with AD.38-43
There was a high number of HS patients who have a clinical history of stroke, also was a greater frequency of white matter changes, a lower frequency of electrocardiogram abnormalities, and diabetes mellitus.37
MTS symptoms include strange sensations, such as auras, euphoria, déjà vu, jamais vu, or fear, Changes in behavior/emotions, Muscle spasms, Temporal Lobe Epilepsy, and Seizures.53
Diagnosis
Neuropsychological tests often reveal signs of impaired functioning of temporal structures, such as poor memory function, or psychiatric disorders such as depression and anxiety. Furthermore, the patient should undergo an imaging scan depending on the clinical history and physical examination if any sign or symptom of MTS is present.53
Imaging Findings
The features of this pathology in diagnostic imaging studies are atrophy of the hippocampus, with an increase in the intensity of the signal on T2-weighted sequences.71
CT scan
The CT scan is helpful in predicting atrophy or other degenerative process that may occur in the medial temporal lobe. In unilateral or bilateral MTS, CT approved efficacy in the diagnosis of cases at a high rate. 70
Some complications may occur after this procedure, such as headaches or seizures. For this reason, medications like diazepam began to be supplied to patients when they returned from CT scans. CT scan is used when there are problems facing patients in using MRI.70
MRI
Neuronal cell loss and gliosis result in the MRI findings of hippocampal atrophy with increased T2/FLAIR signal severity. Secondary signs can be present such as loss of the internal architecture of the hippocampus; loss of hippocampal head digitations; and dilatation of the ipsilateral temporal horn.
The physiologic interconnections between the hippocampus and other parts of the brain can cause secondary signs that can also involve other structures such as increased signal intensity and/or atrophy of the ipsilateral amygdala; atrophy of the ipsilateral mammillary body; atrophy of the ipsilateral fornix; atrophy of the contralateral cerebellar hemisphere; and atrophy of the ipsilateral entorhinal area.60,67,68
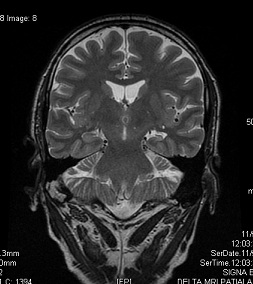
Fig.4 MTS in the left side (coronal T2) 69
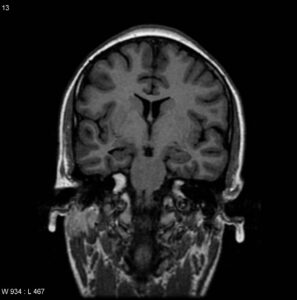
Fig.5 MTS in the right side (coronal T2) 69
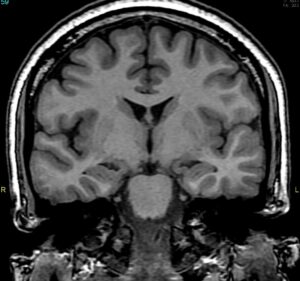
Fig.6 Bilateral MTS (coronal SPGR) 69
Diffusion MRI
The neuronal loss can cause enlargement in the extracellular space and thus diffusion of water molecules is higher on the affected side, resulting in rise values on the affected side (higher signal on ADC). On the other hand, diffusion is limited during a seizure because of neuronal dysfunction and swelling, which explains why values are lower.72
MR spectroscopy
MR spectroscopy findings typically show neuronal dysfunction: Decreased N-acetylaspartate (NAA) and decreased NAA/Cho and NAA/Cr ratios, decreased Myo-inositol (MI) in the ipsilateral temporal lobe and increased lipid and lactate after a seizure.72
MR perfusion
Depending on when the scan is taken, MR perfusion shows similar alterations to SPECT with blood perfusion. During the peri-ictal phases, perfusion is raised, not only in the mesial temporal lobe but also often in large parts of the temporal lobe and hemisphere. In interictal periods, conversely, perfusion is decreased. 7
Nuclear medicine
SPECT (Tc-99m HMPAO or ECD) and PET (F18-FDG) imaging are also helpful adjuncts, with both ictal and interictal scans showing abnormalities: Ictal scan is hyperperfusion and interictal scan is hypoperfusion. 75
Histopathology
HS is characterized by gliosis and neuronal loss, which is most prominent in the CA1 field of the hippocampus, then the hilus, CA3, and CA2 fields, and the dentate granular cell layer.54-57 These changes are commonly accompanied by a dispersion of the dentate granular cell layer, with ectopic neurons being found in the molecular layer.58-59
Also, there are alterations in the expression of a variety of molecules in the surviving neurons, and an axonal reorganization involving both excitatory glutamatergic and inhibitory GABAergic circuits.60
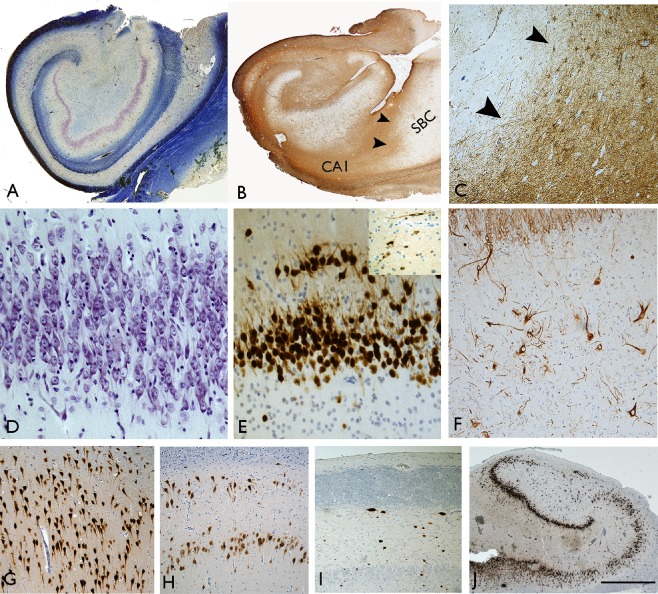
Fig.7 Histopathological Findings in Patients with MTS/HS.1
Complications
MTS is the most frequent cause of temporal lobe epilepsy (TLE). TLE is among all types of epilepsy the most frequently associated with psychiatric comorbidity. Anxiety, depression, and interictal dysphoria are repeated psychiatric disorders in pediatric patients with TLE. As well as, these changes are often combined with cognitive, learning, and behavioral impairment. 62
Mesial TLE with HS is an epileptic syndrome very resistant to pharmacologic treatment, and approximately 50% of patients with this type of epilepsy present drug resistance.63,64
Impairment of the memory system constitutes the most common cognitive deficit in TLE; approximately 70% of patients have problems in episodic memory, associated with the presence of HS.65,66 MTS cause of refractory epilepsy represents between 50% of cases.71
Treatment and management
The treatment of MTS mostly is surgical. 77,79 Patients can be treated medically if they are not candidates for surgery due to extrahippocampal pathology or if surgery is not possible due to financial constraints.83 However, many studies have examined the prognostic factors associated with the success of medical therapy in MTLE results from MTS, finding that such treatment results in completely curable in only 5 to 42% of patients, a percentage much lower than that for other forms of epilepsy.84
Poor prognostic factors include earlier age at onset of epilepsy, bilateral or left-sided lesions, head injury at a young age, EEG abnormalities, and a large number of previously tried anti-epileptic drugs (AEDs).85,86 Although some evidence suggests that adjunctive lacosamide may be successful in the treatment of MTLE, substantial evidence demonstrating the increased relative efficacy of third-generation AEDs in achieving seizure freedom compared with first- and second-generation AEDs has yet to emerge.87
Resistance to drug therapy is a major problem in patients with MTLE, and surgical resection of the affected brain region is a successful treatment strategy in many cases.88 Surgical methods for temporal lobectomy have several technical variations. They involve standard anterior temporal lobectomy, anteromedial temporal lobectomy, selective amygdalohippocampectomy, and stereotactic methods. 76-82 In patients with MTS, anterior temporal lobectomy cures epilepsy in 67% of patients.73
References