Neurology Clinics

Shadowing Program – Neurology Clinic
The Shadowing Program at Neuropedia is the premier initiative in Jordan and the FIRST and Only program catering to medical students, offering an unparalleled experience in the field of Neurology. As a participant, you will have the opportunity to immerse yourself in the Outpatient Clinics of a private Neurology Clinic, where you will gain invaluable insights into various aspects of neurological practice.
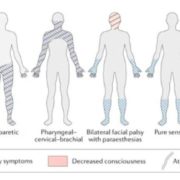
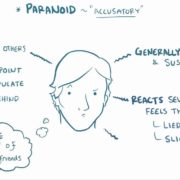
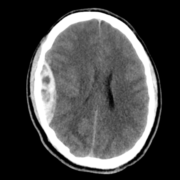
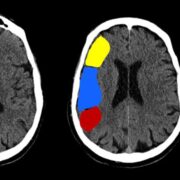
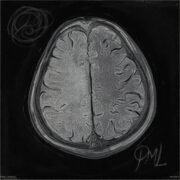
Throughout the program, you will acquire skills such as conducting patient histories, performing neurological examinations, identifying neurological cases, interpreting neuroimaging studies, and much more, all under the guidance of seasoned professionals.
We seek students who harbor a deep passion for neurology, understanding that commitment is fundamental to your journey towards becoming a proficient medical practitioner. To maintain the integrity of our program and uphold professional standards, adherence to the following rules is imperative:
- Punctuality is paramount; complete attendance at clinics is mandatory every day except Friday (6 Days/Week).
- Attire should consist of a lab coat and formal uniform.
- Display your Faculty Badge prominently at all times.
- Prior authorization is required before undertaking any actions involving patients.
Furthermore, at the conclusion of the program, participants will receive a certificate recognizing their dedication and completion of the program. Additionally, there will be a comprehensive evaluation process providing feedback to participants, aiding in their professional development and future endeavors in the field of neurology.
By adhering to these guidelines and actively participating in the evaluation process, you’ll not only enhance your learning experience but also demonstrate your dedication to the field of neurology and your future profession as a doctor.
Duration: One Month
Upon acceptance, you need to pay Registration Fees of 100 JD