Article topic: Central Sleep Apnea
Author: Ihdaa Mahmoud Bani Khalaf
Editor: Hala Qaryouti
Keywords: Apnea, Sleep Disorders, Pathophysiology, Psychiatry, Diagnosis, Treatment
Overview
Central sleep apnea (CSA) is an intermittent repetitive cessation and/or reduction in breathing without respiratory effort, due to an abnormal ventilatory drive (1). It is one of the four sleep-related breathing disorders, which also include obstructive sleep apnea (OSA), sleep-related hypoventilation disorders, and sleep-related hypoxemia disorders (2).
Although almost 5 times less prevalent than OSA(2), CSA accounts for about 5-10% of sleep medicine patients in clinics(1). It has been gaining massive interest in the field of sleep disorders over the last decade due to its excessive reporting in some subpopulations such as patients with heart failure, neurological disorders, or those on high-dose opiates. In addition, the disorder has a challenging nature in its early detection, diagnosis, and suitable treatment (3,4).
Pathophysiology of CSA
Central sleep apnea can be categorized into two main categories; primary (idiopathic) or secondary. The latter is related to Cheyne-Stokes respiration (CSR), medical conditions including chronic renal failure, or high-altitude periodic breathing (5). In addition, central sleep apnea may also manifest during the initiation of treatment of OSA or may occur in association with obstructive sleep apnea-hypopnea syndrome (termed complex sleep apnea) (6). Another CSA subtype can manifest secondary to drugs; as sodium oxybate may promote CSA, while acetazolamide and hypnotics like zolpidem may attenuate the breathing disorder (11).
CSA subtypes are represented by specific pathophysiological mechanisms that trigger certain central breathing patterns which aid in identifying the underlying disease and explaining the context of CSA occurrence. A precise knowledge of pathophysiology is necessary for accurate classification as well as for improving diagnosis and treatment strategies (7).
Another valuable distinction for identifying underlying pathology and deciding the appropriate ventilatory support is waking CO2 levels (8). Therefore, CSA can be also classified into hyperventilation (which is more common) or hypoventilation CSA.
Hypoventilation-related CSA can be seen in conditions where an absent or diminished wakefulness stimulus to breathe causes alveolar hypoventilation(9) via two mechanisms. The first is the inability to translate the central drive to breathe into adequate ventilation due to neuromuscular disorders (e.g. motor neuron disease, poliomyelitis, and neuromuscular junction disorders), and restrictive thoracic cage disorders such as scoliosis. The second mechanism is functional or anatomical lesions at the level of the respiratory centers reducing the ventilatory drive (e.g. congenital and idiopathic central alveolar hypoventilation syndromes, obesity hypoventilation syndrome, medication induced-CSA in severe opioid toxicity, and structural lesions such as brain tumors, Chiari’s syndrome, cerebrovascular diseases). (10)
The underlying mechanism in hyperventilation-related CSA is instability in ventilatory control, as patients have a tendency to develop unstable breathing referred to as high loop gain. This predisposes to hyperventilation and subsequent lowering of PCO2 below the apneic threshold. When carbon dioxide drops below this threshold hypocapnia ensues, and apnea occurs until the carbon dioxide level increases above the threshold. (6,10,12))
Epidemiology and Risk Factors
There are multiple risk factors for CSA such as age, heart failure, male gender, atrial fibrillation, hypocapnia, diuretic use, prior stroke, opioid use and other less prevalent medical conditions such as neuromuscular disease. (3,9,13,14)
According to several studies, the prevalence of CSA increases with aging (13,15). However, according to Young et al.(16) it is uncertain if age alone can be considered an independent risk factor; as older people are more susceptible to developing stroke and heart failure implicated in CSA development. As a result, age may not be a true risk factor.
In regards to gender, according to Bixler and colleagues(14), post-menopausal women have an increased incidence of OSA. However, females in general have an extremely low prevalence of CSA (0.3%) compared to males (7.8%).
In regards to the above-mentioned subpopulations, CSA has a prevalence of 21-37% (for an apnea-hypopnea index (AHI) ≥ 15) in patients with stable congestive heart failure with reduced ejection fraction (3). In patients post-stroke prevalence varies widely between 3-72% (11,17,18).
CSA is also reported in patients on opioid medications, along with OSA patients treated with continuous positive airway pressure (CPAP) so-called treatment-emergent CSA (TE-CSA) (11,19). There are some factors that are identified as predisposing to treatment-emergent CSA (TE-CSA), such as older age, male gender, heart failure, and ischemic heart disease, in addition to the use of high PAP levels, higher residual AHI, central apnea index and arousal index (20,21).
Clinical Presentation
Symptoms of CSA are similar to, yet usually less severe than symptoms of patients with OSA. Patients mainly complain of sleep fragmentation (disturbed sleep cycle due to continuous waking up), and insomnia leading to daytime hypersomnolence (1).
Diagnosis of CSA
A crucial step in CSA diagnosis is differentiation from OSA, which is proving challenging with the emergence of TE-CSA (11).
According to the third International Classification of Sleep Disorders (ICSD-3), full polysomnography (PSG) with esophageal pressure measurement showing absent ventilatory effort during the event is the gold standard for CSA diagnosis (11,18). According to the Diagnostic and Statistical Manual of Mental Disorders- 5th edition (DSM5), the diagnosis requires positive PSG findings as well as the pathology not being better explained by another current sleep disorder (6).
The apnea-hypopnea index (AHI) is the total count of overnight respiratory events (apneas and hypopneas) divided by the total sleep time in hours(22). It is used to classify OSA and evaluate its therapeutic success (23), and also to define TE-CSA; where an AHI >5 events per hour of sleep, more than 50% of which being due to central apneas or hypopneas is used as defining criteria for TE-CSA in adults (11).
If the esophageal pressure measurement cannot be done, surrogates such as strain gauges, respiratory inductive plethysmography (RIP), or belts with piezoelectric or polyvinylidene fluoride (PVDF) sensors can be used(11). RIP is a device that incorporates thoracic and abdominal belts to determine spontaneous tidal breathing without employing a facemask or a mouthpiece (24).
It is worthwhile to say that there is no criterion of desaturation or of arousal required to consider an event as an apnea (25).
Treatment and Management
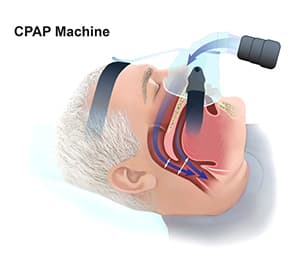
Treatment options for CSA include continuous positive airway pressure (CPAP), adaptive servo-ventilation (ASV), and transvenous phrenic nerve stimulation (26).
According to a randomized clinical trial (RCT) by Bradley et al., CPAP can attenuate central sleep apnea, improve nocturnal oxygen levels, lower noradrenaline levels, increase ejection fraction, and increase the distance a patient can walk in six minutes. However, CPAP does not affect survival in patients with CSA and heart failure.
Another RCT also shows supporting evidence for CPAP potentially improving both left ventricular ejection fraction and heart transplant-free survival in heart failure patients, if CSA is suppressed soon after its initiation. (21, 22)
ASV is mainly used to treat CSA, during this treatment positive airway pressure (PAP) for ventilation support is provided and adjusted as apneic episodes are detected during sleep. Nevertheless, the support provided adapts to the physiology of the patient and can deliver breaths and utilize anti-cyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration (26).
A novel treatment for CSA is transvenous phrenic nerve stimulation which is an implantable device that stimulates the phrenic nerve to initiate breaths and restore a physiological breathing pattern throughout sleep. This therapy stimulates the diaphragm during sleep to stabilize gas exchange and maintain normal breathing.
Recently, temporary transvenous unilateral phrenic nerve stimulation has been shown to result in a more regular breathing pattern, fewer apneic events, improved oxygen saturation, and increased end-tidal carbon dioxide, without suppressing the intrinsic drive to breathe in patients with CSA. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep. (29,30)
Conclusion
As time passes, central sleep apnea is being more recognized due to its frequent incidence among people, especially those with cardiac disease. ASV is mainly used to treat people with CSA and is proving to be a reliable and promising treatment modality that might improve cardiac function, improve sleep quality and possibly confer a survival benefit. Further physiological and clinical research is still required to investigate the central apnea syndromes.
Acknowledgment: We thank IFMSA-JO.
References...