Topic: Idiopathic Intracranial Hypertension (IIH)
Author: Ayah Alatoom
Editor: Ghina Mohamad Kaskas, Ihda Bani Khalaf
Reviewer: Ethar Hazaimeh
Keywords: IIH, papilledema, pseudotumor cerebri, venous sinus stenting, venous sinus stenosis.
Overview
Idiopathic intracranial hypertension (IIH) is a condition of unknown etiology under the Pseudotumor cerebri syndrome (PTCS), previously called benign intracranial hypertension, although the latter term has fallen out of favor due to the morbidity that comes with the condition. PTCS is marked by elevated ICP, normal brain parenchyma, and is classified into primary and secondary PTCS (1). Primary PTCS is the same as IIH, which primarily affects obese young women while secondary PTCS might be due to medications or a systemic medical illness. PTCS is characterized by an elevated intracranial pressure (ICP) producing headaches and visual symptoms that could end in sight loss. Ophthalmic examination is essential and often reveals bilateral papilledema; MRI and lumbar puncture can aid in the diagnosis as well. Treatment is through pharmacological options, including acetazolamide and lifestyle modification, most importantly weight loss, while surgical procedures are preserved for rapidly progressive cases that threaten vision.
Epidemiology
Large epidemiological studies revealed that the incidence of IIH is greatest in obese females of reproductive age and increases significantly in women aged 20 to 44 who are 20% above the recommended body weight, and an estimated 13% of cases occur in men (2). The incidence of IIH differs from one country to another and depends upon the prevalence of obesity in each respective region, as it appears less common in Asian countries compared to the United States. There’s no evidence supporting that IIH has a predilection for a specific racial or ethnic group.
Etiology
As the name implies, there’s no known cause for IIH. Risk factors for the disease include female sex, obesity, and rapid weight gain even in non-obese individuals. Secondary PTCS possible causes include cerebral venous abnormalities like cerebral venous thrombosis, a previous intracranial infection, or subarachnoid hemorrhage that decreases cerebrospinal fluid (CSF) absorption. Several medications may give rise to secondary PTCS, such as tetracyclines, vitamin A and retinoids, lithium, growth hormone, amiodarone, cyclosporine, levothyroxine in children, corticosteroids, danazol, progestin-only contraceptives and combined oral contraceptives. Several systemic medical conditions have been linked to secondary PTCS, such as Addison’s disease, hypoparathyroidism, systemic lupus erythematosus, Turner syndrome, and Down syndrome (1).
Pathophysiology
The precise mechanism by which IIH develops is poorly understood. Some hypotheses were proposed, and there’s a consensus of interplay between three main processes: CSF hypersecretion, obstructed reabsorption, and raised cerebral venous pressure. Moreover, the striking prevalence of IIH in obese women suggests additional metabolic and endocrine factors at play.
CSF is mainly produced by the highly vascularized choroid plexus, at a rate of 500 mL/day, with a total volume of 140-150 mL due to continuous reabsorption, and various choroidal transporters take part in CSF secretion. The ependymal lining of the ventricles, interstitial fluid exchange across the cerebral endothelium, and glymphatic influx from cerebral pulsations play a less important role in CSF secretion (3).
CSF is reabsorbed by the arachnoid granulations, small outpouchings into the dural venous sinuses, that act as one-way valves to provide an outflow for the CSF into the venous sinuses. Lymphatics provide another route; the perineural space of the olfactory nerve bundles is considered a continuation of the subarachnoid space and shares the pressure gradients driving the CSF towards the submucosal lymphatics, where it eventually drains into the cervical lymphatic system. The meningeal lymphatic vessels line the dura matter and drain CSF into the deep cervical lymph nodes (4). The glymphatic system utilizes peri-arterial tunnels formed by astrocytes to both remove waste and distribute compounds across the brain. CSF travels across these peri-arterial spaces to enter the brain interstitium, which is mediated by gaps and channels in the astrocytes’ end-feets. From there, fluid goes into the perivenous spaces and then drains through either the subarachnoid space or the dural lymphatic system. The pressure of the subarachnoid space is greater than that of the cerebral venous sinuses. A pressure gradient of 3-5 mmHg between the cerebral venous sinuses and subarachnoid space is crucial for maintaining unidirectional CSF flow (3).
Understanding CSF dynamics helps explain the pathogenesis of IIH, yet a lot is not known. The pathophysiology of IIH occurs at the intracranial and extracranial levels and involves many factors and players all interacting and influencing each other.
As mentioned, IIH majorly affects obese females. Weight is the only modifiable risk factor in IIH; studies have shown that the disease often resolves with weight loss and recurs with weight gain. The type of obesity observed in IIH patients is truncal, which is non-specific to the disorder and is present in many obese people without IIH (5). Truncal obesity is linked to a pro-inflammatory environment, cardio-metabolic risk, excess glucocorticoids, and excess androgens in women, all of which are connected as one component amplifies many others ending in central fat deposition, thus creating a perpetual cycle of hormonal imbalance and inflammation (6).
Adipose tissue is a source of cytokines, and systemic inflammatory changes occur in obesity. CSF and serum analysis of IIH patients revealed increased cytokines levels in a unique pattern to the disease; this supports an immunological pathway. Leptin appears to be chronically elevated in IIH, this is thought to increase CSF secretion from the choroid plexus through stimulation of the Na+/K+ ATPase pump, a luminal pump that supports active secretion of CSF (7).
IH patients seem to have systemically increased glucocorticoids alongside 11-beta-hydroxysteroid dehydrogenase (11β-HSD), the enzyme responsible for converting the inactive cortisone into cortisol. Activity and expression of 11β-HSD is especially high in adipose tissue, the choroid plexus, and arachnoid granulations also express 11β-HSD, pointing towards a local cortisol-generating system. ICP reduction correlated with reduced serum cortisol:cortisone, and was influenced by 11β-HSD activity (8). Iatrogenic glucocorticoids might increase ICP, even though acute withdrawal from glucocorticoids is associated with raising ICP. Other evidence also suggests that the relationship is not as simple as initially thought, and the extent it plays in IIH pathogenesis stays unclear. Nonetheless, glucocorticoids are dysregulated in IIH.
In clinical studies, serum testosterone and 5-alpha-reductase as well as CSF testosterone and androstenedione, were found elevated in IIH. The choroid plexus expresses androgen receptors and activating enzymes and rat models showed increased Na+/K+ ATPase pump activity in response to androgen administration (9). Moreover, the pattern of androgen elevation in IIH seems to be distinct from that of PCOS, a common comorbidity in women with IIH, and higher androgen levels appear to correlate with earlier disease onset (10). Potent androgens seem to be either activated in the choroid plexus and/or cross the blood-brain barrier after activation in other tissues, where they affect CSF secretion and ICP. Still, we cannot say androgens excess is the main driver of IIH, especially since the condition occurs in males —interestingly, it was reported in the setting of hypoandrogenism— further complicating our understanding of the disease (11).
These metabolic and hormonal factors were proposed to try to explain the process behind IIH, yet a lot remains unknown to us. Nevertheless, they appear to dysregulate CSF dynamics, primarily by driving its secretion.
Going back to obesity and its role in IIH pathogenesis. An old suggestion was that an increased intra-abdominal pressure translates to an increased intracranial pressure. However, this does not explain why most obese people do not suffer from IIH. Even so, transverse sinus stenosis is a common finding on imaging studies, indicating increased venous outflow resistance. This is likely secondary to the high ICP, but it still contributes to progression by initiating a positive feedback loop; venous congestion reduces CSF absorption, further increasing ICP (12).
Finally, the newly discovered glymphatic system seems to be dysfunctional in IIH. Pathological changes in the interface between glial cells, vasculature, and neurons of the glymphatic system were noted that possibly disrupt the blood-brain barrier, allowing for pro-inflammatory substances to leak into the brain tissue (13).
Clinical Presentation
The presentation of IIH is highly variable, which could delay diagnosis and appropriate treatment. A landmark study of the disease, The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), involved 317 participants, with 98% being women. The reported symptoms included headache (84%), transient visual obscurations (68%), pulsatile tinnitus (52%), dizziness (51%), photophobia mostly accompanying the headaches (48%), visual loss or blurring (32%), radicular pain (19%), and double vision (18%) (14).
Headaches are the most frequently reported complaints and are highly heterogeneous. They’re described as pressure-like, sometimes throbbing or pulsatile, and are often focal; retro-orbital, holocranial, occipital, temporal, or frontal, and might be accompanied by nausea, photophobia, and phonophobia. In many cases, it resembles chronic migraines, which could co-exist in this demographic (15). Headaches typically worsen with activities that raise intracranial pressure, such as straining, bending forward, lying supine, or performing the Valsalva maneuver and show transient improvement following a lumbar puncture (LP). The pain is usually daily or constant, significantly affecting daily functioning and quality of life (16). This often leads to frequent analgesic use, increasing the risk of medication-overuse headaches, which can further complicate the clinical picture.
Pulsatile tinnitus is another common problem in IIH, more often bilateral than unilateral, and occurs every 2 days. The tinnitus improves with pressure on the ipsilateral jugular vein, and it’s thought to arise from flow turbulence in the stenosed transverse venous sinus.
Transient, unilateral, or bilateral visual obscurations (TVO) lasting less than a minute are noted with postural changes, arising after bending over, or from eye movement. Elevated local tissue pressure interrupts the microcirculation of the optic nerve, resulting in transient ischemia. TVOs are nonspecific to IIH and do not correlate with the degree of disc edema.
Diplopia is another visual complaint, often horizontal and binocular, and although increased ICP, in general, is linked to abducens nerve palsy and diplopia, only a minority of IIH patients with diplopia in the IIHTT had abducens palsy, hinting toward an alternative cause.
Progressive, insidious visual field loss can begin early in the disease with enlargement of the blind spot or field defects, arcuate or nasal. Elevated ICP transmits via the subarachnoid space around the optic nerve, causing axoplasmic flow stasis and optic disc swelling –papilledema (17). The visual field defects relate to the degree of papilledema unlike TVOs and are often reversed with treatment. Peripapillary elevation of the retina from papilledema may contribute to hyperopic defocus in the elevated area.
Interestingly, blind spot enlargement in IIH patients improves with the addition of plus lenses (18). In advanced stages, fluid tracking from the disc to the fovea may lead to neurosensory retinal detachment, causing irreversible loss of visual acuity, or this fluid may result in choroidal or retinal folds, contributing to refractive disturbances (19). The degree of papilledema varies among patients and is identical to the papilledema seen in brain tumors and other space-occupying lesions. Papilledema is frequently bilateral and symmetrical, while asymmetrical disc swelling accounted for 7% of the cases in the IIHTT. Acute papilledema is marked by a hyperemic disc, accompanied by flame hemorrhages, hard exudates, and venous engorgement while chronic papilledema is seen with telangiectasias, disc pallor, and optocilliary shunts (17). The visual outcomes in IIH depend upon the onset of the visual disturbances and their severity at presentation, and an estimated 10-24% of patients end with permanent vision loss(18) .
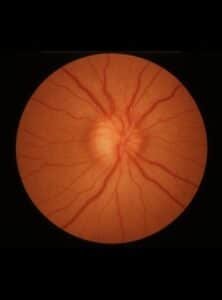
Figure 1 Papilledema – Optic disc swelling due to increased intracranial pressure.
Uncommon symptoms of IIH include cognitive impairment, most pronounced in reaction time and processing speed, and unfortunately, can persist despite lowering the ICP (20). Pain involving the neck and shoulder in a radicular or dermatomal pattern, fourth and third cranial nerve palsies, and facial nerve and trigeminal nerve dysfunction have also been reported as rare, atypical symptoms. CSF may leak in the form of rhinorrhea from defects in the cribriform plate and a resultant dural breach, causing paradoxical low-pressure symptoms (21).
Comorbid conditions in patients with IIH include PCOS and anemia. PCOS in IIH is common (20%) and is associated with higher infertility, but does not exacerbate long-term vision or headache prognosis (22). Anemia, typically iron-deficiency anemia, is 44% more common in patients with IIH. Pooled data suggest an 18.2% prevalence of anemia among IIH patients, and IIH in anemic patients spans all ages, unlike typical IIH (usually young, obese women) (23). This is in part due to a demographic overlap of females of child-bearing age, but it’s not totally incidental as the ICP declines after correcting the iron deficiency.
Strong associations have been established between IIH and features of metabolic syndrome, including central obesity, insulin resistance, hyperleptinemia, androgen excess, and increased long-term cardiovascular morbidity, with elevated risks of hypertension, ischemic heart disease, and stroke that persist even after adjusting the body mass index (24). Suggesting a pathogenesis independent of adiposity.
The clinical presentation of IIH in men seems to be different, as at their first presentation, they’re less likely to complain of headaches and continue to report fewer headaches at subsequent follow-up visits, but rather present with visual disturbances and are twice as likely as women to develop severe visual loss (25). IIH was found to have a greater incidence of obstructive sleep apnea (OSA), reaching 50%, and it’s more frequent in men with IIH compared to women (26).
Workup and Diagnosis
IIH is primarily a clinical diagnosis, supported by imaging studies and lumbar puncture. Diagnostic criteria have changed several times since the Dandy criteria were first published in 1937. The latest rendition of the criteria, the revised 2013 Modified Dandy Criteria (Table 1), included the presence of papilledema along with other existing criteria for a confident diagnosis of IIH (1). The criteria also enabled a diagnosis of IIH without papilledema to be made, but require typical features of the disease, including abducens nerve palsy, and additional imaging findings. If papilledema and abducens nerve palsy are both absent, a diagnosis can be suggested based on other findings.
Table 1 Revised 2013 Dandy Criteria for Pseudotumor Cerebri (1)
|
|
|
|
|
|
|
| In the absence of papilledema, a diagnosis of pseudotumor cerebri syndrome can be made if B–E from above are satisfied, and in addition, the patient has a unilateral or bilateral abducens nerve palsy |
| In the absence of papilledema or sixth nerve palsy, a diagnosis of pseudotumor cerebri syndrome can be suggested but not made if B–E from above are satisfied, and in addition at least 3 of the following neuroimaging criteria are satisfied: |
|
|
|
|
| A diagnosis of pseudotumor cerebri syndrome is definite if the patient fulfills criteria A–E. The diagnosis is considered probable if criteria A–D are met, but the measured CSF pressure is lower than specified for a definite diagnosis. |
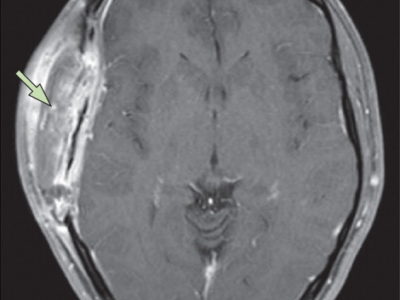
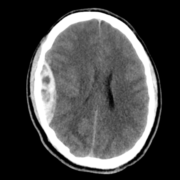
Imaging studies are essential to confirm the diagnosis of IIH after being suspected clinically. Magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) are the modalities of choice. Computed tomography (CT) is helpful as a less expensive, quicker method for ruling out space-occupying lesions, but MRI and MRV remain superior as they provide more accurate findings. On imaging of the orbits, optic nerve sheath distention is the most common finding, posterior globe flattening, tortuosity of the optic nerve in vertical or horizontal planes, optic nerve head enhancement, and papilledema in the form of optic nerve head protrusion are also observed. Enlarged arachnoid outpouchings in the form of an empty or partially empty Sella turcica, enlarged Meckle cava, oculomotor cisterns, perivascular spaces, and arachnoid pits/small meningoceles while venous outflow obstruction could take place in the form of transverse sinus stenosis or internal jugular vein stenosis (27).
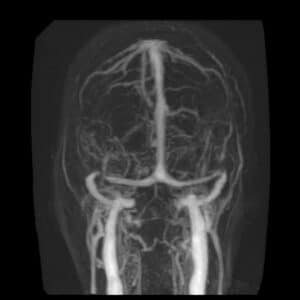
Figure 2 MR venography revealing focal stenosis of the lateral aspect of both transverse sinuses.
Acquired cerebellar tonsillar ectopia and slit-like ventricles are other neuroradiological findings. “Empty” sella, posterior pituitary stalk displacement, meningoceles, posterior globe flattening, optic nerve head protrusion, optic nerve enhancement, optic nerve sheath distension, optic nerve tortuosity, slit-like ventricles, tight subarachnoid spaces, and inferior position of cerebellar tonsils have overall high specificity but low sensitivity and are considered supportive rather than diagnostic of the disease thus should always be interpreted in the context of clinical symptoms and elevated lumbar puncture opening pressure. Transverse sinus stenosis appears to be the most useful sign because it has high specificity and fairly high sensitivity (27).
After excluding a space-occupying lesion with imaging studies, a lumbar puncture should be carried out in a relaxed lateral decubitus position to avoid false values. An opening pressure above 25 cm H2O is deemed diagnostic in adults, but in children, the normal opening pressure tends to be higher (12-28 cm H2O). Sedation is avoided when performing the lumbar puncture since it can result in hypercapnia, which artificially increases the opening pressure. If the opening pressure is below the threshold but the patient exhibits other disease characteristics, this should not negate the diagnosis as the opening pressure reflects the CSF pressure at that given moment, a repeat Lumbar puncture or continuous pressure monitoring helps in establishing a more accurate reading but could be uncommon and impractical (28). Many patients report a transient improvement in their headaches after the lumbar puncture, but this is considered subjective and not diagnostic. Other constituents of the CSF should be recorded and are expected to be normal, but low protein might be present.
Visual fields and optic fundi should be checked in all patients, even in the absence of visual symptoms. Optic coherence tomography (OCT) provides objective quantification of papilledema and characterizes changes in the retinal nerve fiber layer (RNFL). OCT can show increased thickness of the RNFL in IIH patients, which correlates with visual field values at diagnosis (29).
Routine blood work is not required for a definitive diagnosis of IIH, but could help rule out other differential diagnoses, identify secondary causes of increased ICP, assess associated or comorbid conditions, and evaluate the overall health of the patient before treatment.
A comprehensive cardiovascular and metabolic assessment is advised regardless of body mass index, as recent studies showed an increased risk of ischemic stroke/TIA, non-traumatic hemorrhagic stroke, elevated risks of insulin resistance, and type 2 diabetes mellitus (24).
Management
The main aims of treatment in IIH include pressure reduction, preservation of vision, and symptom control. If appropriate, weight loss should be a cornerstone of management. In one prospective cohort trial, 25 women with chronic IIH were treated with a very low-calorie diet (425 kcal per day) for 3 months; patients lost 15% of their body weight, resulting in a significant reduction in ICP, headaches, and papilledema. Aided weight loss with bariatric surgery or GLP-1 agonists might markedly improve the disorder for obese patients who were otherwise unable to lose weight. Low-sodium diets should be promoted, and any medication known to increase the ICP should be discontinued or avoided (30).
Pharmacotherapy is the first-line management in the absence of fulminant visual loss. Acetazolamide affects CSF production at the choroid plexus by inhibiting carbonic anhydrase, an enzyme that converts water and carbon dioxide to bicarbonate and hydrogen ions, affecting the efficacy of ion transporters. The IIHTT (14) compared acetazolamide with a placebo and both groups received a supervised low-salt weight-reduction diet and a lifestyle modification program; the grade of papilledema, CSF pressure, visual quality of life, general quality of life, and weight loss measures were all significantly better in the acetazolamide group. The side effects of acetazolamide include nausea, vomiting and dysphagia which could contribute to weight loss, paresthesia, and fatigue are observed as well.
Topiramate, originally used as an anti-seizure and migraine prophylactic medication, is a promising medication as it has weak carbonic anhydrase inhibiting effects and suppresses appetite providing ICP reduction, headache control, and weight reduction. When compared with acetazolamide, topiramate seems to harbor similar effects with no difference in outcomes, but prominent weight loss was noted with topiramate. Its adverse effects are like acetazolamide’s: paresthesia, fatigue, and gastrointestinal upset, but cognitive effects can happen, especially at higher doses, which is undesirable in a population with growing evidence of cognitive risk (31).
Furosemide is a loop diuretic that is uncommonly used alone but sometimes combined with acetazolamide in resistant cases and showed favorable outcomes in a pediatric case series (32).
IIH patients experience headaches that share many similarities with migraine; as such, symptomatic treatment for episodic headaches includes many migraine prophylactic drugs like amitriptyline, propranolol, CGRP (calcitonin gene-related peptide) antagonists, topiramate non-steroidal anti-inflammatory drugs (NSAIDs). Patients should be instructed to avoid overuse of headache medications as their condition might be further complicated by medication-overuse headaches (33)
Serial lumbar punctures for disabling headaches can be done but have fallen out of favor since CSF reforms within 6 hours. Most patients experience small, short-lived relief, which tends to be more pronounced in those with severe baseline headaches. However, there is a significant risk of developing post-lumbar puncture headaches exacerbation, possibly due to over-drainage or fluctuations in ICP (34). Serial LPs can be used in urgent cases where there’s rapid deterioration of vision loss ,as a definitive surgical option is prepared
Regular assessments for vision with monthly to quarterly follow-ups are crucial for IIH, especially quantitative visual field tests as simple visual acuity exams can’t detect early signs of vision loss.
Cases refractory to medical treatment or with rapid progressive loss of vision occur like Fulminant IIH —a severe and rapidly progressing form of IIH marked by acute vision loss within four weeks of symptom onset, are treated differently than the usual chronic cases of the disease. In such situations, surgical options are preferred, aiming to lower the ICP and preserve vision. Such options include optic nerve sheath fenestration (ONSF), CSF shunting, venous sinus stent placement, or placing a temporary lumbar drain until a surgical plan is decided upon (35). The procedure performed is chosen based on availability, with no strict recommendations, due to the lack of evidence-based trials and guidelines on surgical interventions. ONSF involves cutting a window into the optic nerve sheath to allow CSF to escape when vision is the main concern, but provides the least headache relief, while CSF diversion procedures include Ventriculoperitoneal (VP) or Lumboperitoneal (LP) shunts to provide relief for severe headaches but have high rates of complications (36). Venous Sinus Stenting can be considered for severe stenosis of the transverse sinus and shows improvement in pressure gradients. It’s a promising option, but Long-term data are scarce, and it’s still considered off-label in many regions. A recent prospective study utilized a specially designed stent for IIH sinus stenosis. It enrolled 39 patients who had failed medical therapy and reported positive 1-year outcomes (37).
Prognosis
IIH is not associated with a significant mortality risk, and the morbidity of the disease comes from headaches or visual impairment. Headaches are increasingly recognized as a cause of long-term disability (16). Chronic papilledema causes ischemic damage to the optic nerve fibers and optic nerve atrophy, which can end in visual field constriction, reduced acuity, and loss of color vision. Timely and appropriate treatment of IIH can prevent these adverse outcomes and provide an encouraging prognosis. The risk of vision loss is greater in males, patients with early onset of reduced visual acuity, black ethnicity, and appears unrelated to TVOs, degree of papilledema, and number of recurrences (18). The recurrences happen with weight gain, putting further emphasis on the importance of weight loss and lifestyle changes in the management plan (38). Good prognosticators include a young age of disease onset and high diagnostic ICP, as they’re linked to less diagnostic latency.
Recent Updates
Venous Sinus Stenting: The RIVER Trial
The RIVER trial is the first prospective multicenter study evaluating a stent specifically designed for intracranial venous sinuses (37). It enrolled 39 patients with IIH who had severe headaches or visual field loss and had failed medical therapy. The study reported positive 1-year outcomes; 79% experienced reduced headaches, 89% improvement in papilledema, 88% relief in diplopia or visual disturbances, and a major complication rate of 3.93% and a minor complication rate of 2.72% post-venous sinus stenting. These findings support the use of venous sinus stenting as a minimally invasive and safe surgical option, especially for patients who didn’t benefit from medical treatment.
Metabolic Dysfunction and Biomarkers
It is increasingly recognized that IIH is not solely a central nervous system disorder and has systemic and metabolic features (39). A prospective case-control study collected cerebrospinal fluid, serum, and clinical data from 60 new-onset, treatment-naïve IIH patients, identifying novel biomarkers; S1P and LysoPC-16 showed potential prognostic value due to association with subsequent optic nerve atrophy.
Cardiometabolic Risks in IIH
A retrospective analysis of electronic health records from 2009 to 2024 investigated the cardiometabolic outcomes associated with IIH (24). The study found that IIH patients had increased risks of cardiovascular and metabolic conditions which persisted in non-obese IIH patients. This study adds to the notion that IIH is a complex multi-system disorder with cardiovascular and metabolic implications and highlights the importance of comprehensive cardiovascular and metabolic screening in IIH patients, regardless of BMI status.
Pediatric IIH Consensus
Recognizing the lack of national guidance on pediatric IIH, a Delphi consensus via the Children’s Headache Network proposed a best-practice diagnostic and therapeutic pathway for children with IIH (40). 104 questions were considered by 66 physicians consisting of general pediatricians, pediatric neurologists, ophthalmologists, opticians, neuroradiologists, and neurosurgeons with a clinical interest or experience in IIH. These questions addressed important aspects of IIH care: assessment, diagnosis, treatment, follow-up, and surveillance to provide a basis for a pragmatic