Article Topic: Trigeminal Autonomic Cephalalgias (TACs)
Author name: Sara Shatnawi
Keywords: cluster headache, hemicarnia continua, paroxysmal hemicarnia, nVNS, SUNCT.
Overview
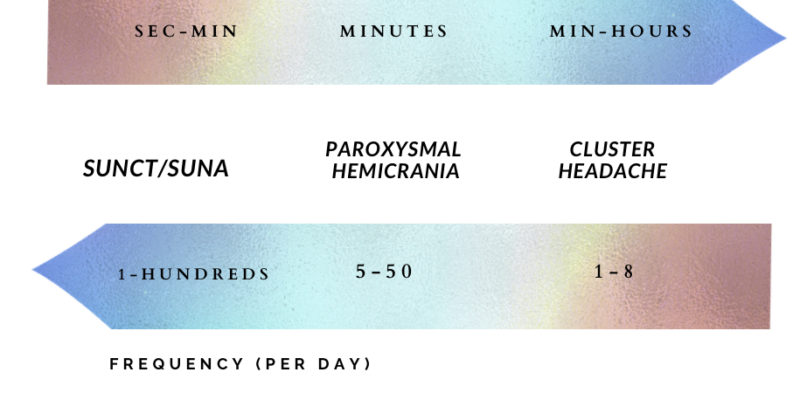
Trigeminal autonomic cephalalgias (TACs) are a group of primary headache disorders which include cluster headache, paroxysmal hemicrania (PH), hemicrania continua, short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT), and short-lasting unilateral headache with cranial autonomic symptom (SUNA). All TACs share common features of recurrent unilateral pain and are often accompanied by ipsilateral cranial autonomic features such as ptosis, conjunctival injection, lacrimation, nasal congestion, or rhinorrhea. The duration of the attacks, their frequency, and the way the headache responds to treatment differentiate between each type. SUNCT and SUNA are the most frequent but their attacks lie for a short duration of time, inversely, cluster headache has the lowest attack frequency, but often the longest duration. Paroxysmal hemicrania lies at the midpoint of the spectrum; it occurs more frequently than cluster headache but significantly less than SUNCT/SUNA, with a duration between the two. (1)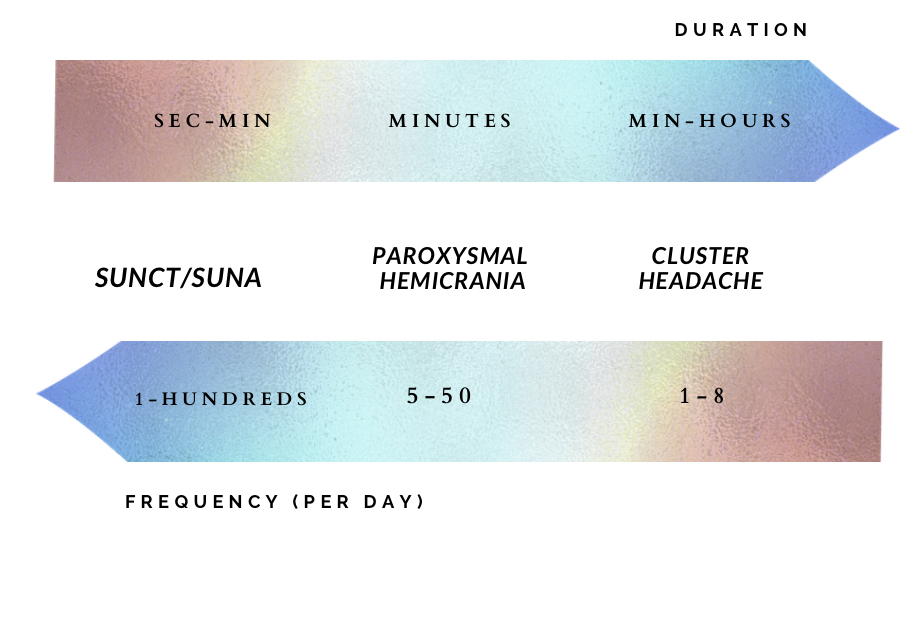 Figure 1. Typical durations and frequencies of individual attacks of Trigeminal autonomic cephalalgias per day.
Figure 1. Typical durations and frequencies of individual attacks of Trigeminal autonomic cephalalgias per day.
Pathogenesis and Etiology
The three brain systems involved in TACs are the trigeminovascular system, the autonomic system, and the hypothalamus (2).
- The trigeminovascular system: facial pain system. The ophthalmic branch of the trigeminal nerve receives input information from the forehead, eyes, dura and large cranial nerves. The trigeminal nerve connects to several nociceptive nuclei in the brainstem and upper cervical cord also called trigeminocervical complex, which then reaches the thalamus, and finally the pain neuromatrix (a collection of areas in the brain that processes many types of pain) (3). During a cluster attack, the trigeminovascular system elevates several signaling molecules including calcitonin gene-related peptide (CGRP) (3, 4).
- The autonomic system: responsible for cranial autonomic features (lacrimation, conjunctival injection) which is a series of parasympathetic over-activation and sympathetic inactivation. The superior salivary nucleus provides parasympathetic input to the sphenopalatine ganglion, which in turn supplies the face, specifically the lacrimal gland and paranasal sinuses. The most direct evidence of autonomic nervous system involvement is achieved by placing an electrode over the sphenopalatine ganglion in patients with cluster headaches, the attack can be exaggerated or relieved by changing the stimulation parameters (5). Vasoactive intestinal peptide is a signaling molecule in the autonomic nervous system that is elevated during a cluster attack (3, 4).
- The hypothalamus: includes the circadian system and aggression areas, which may explain the predictable daily rhythmicity of the cluster headache and restlessness seen in patients with TACs. Furthermore, molecules regulated by the hypothalamus, such as melanin, are altered in patients with cluster headaches (6). The hypothalamus appears to be the first area activated during a cluster attack, followed by trigeminovascular and autonomic activation. Genetics may play a role in the occurrence of all types of TACs (3).

Figure 2: Pathophysiology of cluster headache initiation. (38).
Cluster Headache
Cluster headaches have a male predominance of approximately 72% (7), with an age of onset between 20 and 40 years (8). In addition, its prevalence is 0.1% of the general population (9).

Figure 3: Patient with cluster headache during a right-sided painful attack. note the ipsilateral ptosis, miosis, lacrimation and rhinorrhea. (36).
Clinical presentation
The criteria for cluster headache are shown in Table 1. (37)

There are two types of cluster headache based on (ICHD-3) Diagnostic Criteria for Cluster Headache:
- Episodic cluster headache: Attacks fulfilling criteria for cluster headache and occurring in cluster periods with at least two lasting from 7 days to 1 year when untreated, separated by pain-free periods that last for more than 3 months.
- Chronic cluster headache: Attacks fulfilling criteria for cluster headache and occurring without a remission period, or with pain-free periods lasting less than 3 months for at least 1 year. (2, 3)
Cluster headaches are diagnosed according to the patient’s description of pain. Patients complain of unilateral orbital or temporal pain, the onset and cessation of the attack is sudden (11). In addition, the pain reaches its maximum intensity over 5 to 15 minutes (3). The most distinctive feature of this type of headache is its clocklike regularity reported in 82% of the patients. Moreover, it can be triggered by many factors including alcohol, nitroglycerin, heat, high altitude, exercise, and strong scents such as solvents and cigarettes.
Management
Treatment of cluster headaches includes a combination of acute, transitional, and preventative medications. Additionally, lifestyle modifications can be encouraged to avoid any triggering factors such as alcohol (3).
- Acute treatment: patients with an acute attack can be treated by high-flow oxygen and triptans. Oxygen should be administered at 100% via a nonrebreather mask at a rate of 12-15 L/min for at least 20 minutes. Triptans have several routes of administration including subcutaneous, oral, and nasal, with subcutaneous being the most effective, followed by nasal and oral. However, for patients who encounter multiple attacks per day or who are unable to take the full dose of Triptan each month, the safest treatment is oxygen and noninvasive vagus nerve stimulation. Nasal lidocaine and oxygen can be given to pregnant and breastfeeding women (15).
- Transitional medications: the mainstay of short-term prophylaxis is greater occipital nerve block with local anesthetic and steroids, or a course of oral steroids. Oral steroids are then tapered over three weeks to avoid potential side effects such as osteonecrosis of the hip, especially when used for a prolonged period. The use should be limited to 2 to 3 courses per year.
- Preventative medications: cluster headache can be prevented using verapamil. A typical daily maintenance dose is 480 mg to 720 mg, divided into 3 doses. High doses of verapamil should be avoided because of its ability to cause cardiac conduction abnormalities, specifically elongation of the PR interval. Therefore, ECG monitoring is required as a baseline investigation (before initiating the first dose), 10 to 14 days after each dose, and every 6 months whilst on medication. (3,11)
Other medications that have been shown to be helpful for cluster headache are topiramate, lithium, Melatonin, baclofen and valproic acid.
- Refractory cases: a sleep study and pituitary laboratory studies should be considered, as well as invasive approaches. Neuromodulation is generally preferred because it’s minimally destructive. Sphenopalatine ganglion stimulation, occipital nerve stimulation, and deep brain stimulation of the hypothalamus are invasive neuromodulation treatments for cluster headache. (3)
Patients with cluster headaches will acquire substantial but short-lasting relaxation from subcutaneous sumatriptan and high-flow oxygen, if it is not potent then indomethacin is used to rule out other TACs. In addition, brain MRI with pituitary and cavernous sinus view is the workup required for patients diagnosed with cluster headache according to European Headache Federation consensus (12). A magnetic resonance angiogram (MRA) of the head and neck can be done for patients who don’t respond to verapamil. In some patients erythrocytes sedimentation rate may be required to rule our temporal arteritis or referral to ophthalmology, dentistry, or otolaryngology may be appropriate. (3, 13, 14)
Paroxysmal Hemicrania
Paroxysmal hemicrania is less common compared to cluster headaches, and more prevalent in women. Its overall rate is 0.5 per 1000 or less, with an age of onset between 30 and 40 years. Patients with paroxysmal hemicrania presents with a severe unilateral headache. The pain is often described as throbbing, sharp, or stabbing pain localized around or behind the eye, with a similar intensity to cluster headache. It usually occurs more than 5 times a day. Furthermore, the pain is associated with ipsilateral cranial autonomic features and/or restlessness. Lacrimation is the most common autonomic symptom, as well as restlessness (16). It may be associated with migraine-like symptoms including photophobia ipsilateral to the pain and vomiting (2).
Clinical presentation

Management
Paroxysmal hemicrania attacks are too short for acute medications, thus management is focused on prevention.
The treatment begins with indomethacin, starting with a dose of 25mg taken 3 times a day over 1 to 2 weeks. The dose is then stepped up to 75mg, 3 times a day for 1 to 2 weeks (17). NSAIDS like Indomethacin have a high risk of causing gastrointestinal ulcers, so it is given along with proton pump inhibitors or histamine receptor 2 antagonist (h2 blockers). Indomethacin can also increase risk of cardiovascular events, kidney toxicity and bleeding. Usually, patients report a new type of headache after starting the treatment (18). Once the headache improves, women of childbearing age should stop indomethacin before pregnancy. If indomethacin is not effective other causes should be ruled out. For patients who can’t tolerate indomethacin, cyclooxygenase type 2 inhibitors, verapamil, and topiramate can be used. Sphenopalatine ganglion and greater occipital nerve blocks have also been useful in treating paroxysmal Hemicrania (3, 19)
SUNCT and SUNA
SUNCT and SUNA are somewhat more common in men and have a prevalence of 0.05 to 1 in 1000, the age of onset is older in comparison with other TACs, and thus it’s found in people aged between 40 to 70 years.
Patients with SUNCT and SUNA typically present with unilateral headache with orbital, supraorbital, temporal, and/or other trigeminal distribution of moderate to severe intensity. Attacks typically last from 1 to 600 seconds, occurring as single stabs, series of stabs, or in a saw-tooth pattern at least once a day and associated with ipsilateral cranial autonomic features.
The SUNCT and SUNA have similar clinical features. To differentiate between them, patient with SUNCT present with both lacrimation and conjunctival injection, while patients with SUNA may present with one or neither. Triggers are touching the area of pain, chewing, or brushing the teeth (2, 20). Work up includes brain MRI and arterial imaging of the head and neck (12). Trigeminal nerve view can also be evaluated (3).
There are two different types of SUNCT and SUNA:
- The episodic type: patients have 2 episodes often lasting from 7 days to 1 year if left untreated, and pain remission periods of more than 3 months.
- The chronic type: occurs with no remission periods or with remission periods lasting lesser than 3 months in 1 year.
Management
SUNCT and SUNA are mainly treated with Lamotrigine, taken daily with a dose ranging between 100 mg to 200 mg. The second line treatment is topiramate or gabapentin. Patients who require short-term relief can be given steroids. However, the most effective treatment is IV lidocaine given at a rate of 1 to 3.5 mg/kg/h, but IV lidocaine should be avoided in patients with cardiac conduction abnormalities. The typical protocol involves 1 week admission with continuous monitoring of the vital signs. (3)
Hemicrania continua
Hemicranias continua true prevalence is unknown, since only few cases have been reported, but it’s more common in women and is found in 0.8% of the patients who complain of day-to-day headaches.3
Hemicrania continua is characterized by unilateral frontal or temporal pain that’s throbbing in nature. Patients often describe the pain as a foreign body or itchy sensation of the eye. The intensity of the pain is mild or moderate. Moreover, Headache exacerbation often lasts minutes to days and are accompanied with ipsilateral cranial autonomic features as well as nausea, vomiting and photophobia (3). Triggers are alcohol, stress and irregular sleep (11).
Hemicrania continua is divided into two types, remitting and unremitting (7):
- Remitting: the headache is not daily or continuous and has remission periods of more than 24 hours (without treatment).
- Unremitting: headache is daily, ongoing, and has no remission periods more than 24 hours.
Patients with hemicrania continua are followed up with brain MRI and arterial imaging of the head and neck using MRA to evaluate structural causes of chronic daily headache (12). Moreover, venous imaging using magnetic resonance venogram (MRV) is done to rule out venous sinus thrombosis, and erythrocytes sedimentation rate (ESR) is measured to exclude temporal arteritis (3).
Management
The first-line treatment of hemicrania continua is indomethacin, the starting dose is 225 mg/d for 2 weeks followed by down-titration to find the minimum effective dose for the patient. Gastro-protective agents should be prescribed as well. For patients whom indomethacin is contraindicated, other medications such as celecoxib, topiramate, gabapentin, and melatonin have been suggested as second or third-line treatment (21). Melatonin can be used as an adjunct to decrease the dosage of indomethacin (22). Effective treatment with occipital nerve blocks and onabotulinum toxin have also been reported. For refractory patients, occipital nerve stimulation as well as radiofrequency of the sphenopalatine ganglion may be helpful (2).
Recent updates
- In a recent placebo-controlled trial, the calcium gene-related peptide (CGRP) monoclonal antibody galacanezumab has shown effectiveness in the prevention of cluster headaches. (23)The study involved 106 participants, 49 of them received galacanezumab and 57 of them received a placebo. During the study the patients’ vital signs, electrocardiograms and laboratory measures were monitored to avoid any potential side effect. Galacanezumab recipients’ experienced significant reduction in the weekly frequency of attacks from week 1 to week 3 compared with placebo. 71% and 53% in the galcanezumab and placebo groups, achieved 50% reduction in headache frequency respectively. (24)
- Sodium oxybate has been evaluated for the prophylaxis of cluster headaches in a small open labeled study involving four patients (25). The results proved a long lasting improvement in frequency and intensity of both nocturnal and daytime headaches. Additionally the treatment is dose-dependent. (26)
- The effectiveness of Psilocybin and Lysergic Acid Diethylamide (LSD) for the treatment of cluster headache is not well known. 53 patients were enrolled in a case study that showed promising results 25 for LSD as a prophylactic treatment and psilocybin as both a prophylactic and acute treatment. (27)
- Deep brain stimulation of the posterior hypothalamus was recently practiced to treat the chronic cases of cluster headaches (28). It was also found to be effective in three cases diagnosed with SUNCT (29, 30). The surgery showed an evident and absolute decrease in attack frequency. However, the useful outcome of the surgery is not immediate and requires variable period of 1 to 4 months to be apparent. The post-operative side effects included diplopia (31), vertigo (32), and a rarely intracerebral hemorrhage which can subsequently result in death (33, 34).
- Other new surgical methods has been identified for the treatment of SUNCT and SUNA including microvascular decompression of the trigeminal nerve (9). Cases diagnosed with SUNCT and SUNA unresponsive to medical therapy, agreed on following surgical treatment, MRI imaging of their brain revealed an abnormal arterial loop compressing the trigeminal nerve. The end result of surgery was 67% of the patients were completely relieved during the follow up period of 9 to 32 months and 33% of the cases didn’t improve. This concludes that patients with chronic SUNCT and SUNA with an abnormal vascular loop may receive benefit from surgery. (34)
- A study in the UK was done to evaluate the effectiveness of noninvasive vagal nerve stimulation (nVNS) in the treatment of cluster headache for patients who are unresponsive or cannot tolerate multiple preventative medications or acute treatments. 30 patients participated in the study, out of which 29 had chronic CH and 1 patient had episodic CH. The results have shown a notable decrease in the attack frequency, duration and severity, and no serious side effects, thus demonstrating the efficacy and safety of nVNS in CH. (35)
References
- Newman LC. Trigeminal Autonomic Cephalalgias. www.ContinuumJournal.com
- Burish MJ, Rozen TD. Trigeminal Autonomic Cephalalgias. Neurologic Clinics. 2019;37(4):847-869. doi:10.1016/j.ncl.2019.07.001
- Burish M. Cluster Headache and Other Trigeminal Autonomic Cephalalgias. 2018. http://journals.lww.com/continuum
- Goadsby PJ, Edvinsson L. Human in Vivo Evidence for Trigeminovascular Activation in Cluster Headache Neuropeptide Changes and Effects of Acute Attacks Therapies. 1994;117. http://brain.oxfordjournals.org/
- Schytz HW, Barløse M, Guo S, et al. Experimental activation of the sphenopalatine ganglion provokes cluster-like attacks in humans. Cephalalgia. 2013;33(10):831-841. doi:10.1177/0333102413476370
- Waldenlind E, Ekbom K, Wetterberg L, et al. Lowered circannual urinary melatonin concentrations in episodic cluster headache. Cephalalgia. 1994;14:199-204.
- Rozen TD, Fishman RS. Cluster Headache in the United States of America: Demographics, Clinical Characteristics, Triggers, Suicidality, and Personal Burden. Headache. 2012;52(1):99-113. doi:10.1111/j.1526-4610.2011.02028.x
- Manzoni GC, Taga A, Russo M, Torelli P. Age of onset of episodic and chronic cluster headache – a review of a large case series from a single headache centre. Journal of Headache and Pain. 2016;17(1). doi:10.1186/s10194-016-0626-9
- Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: A meta-analysis of population-based studies. Cephalalgia. 2008;28(6):614-618. doi:10.1111/j.1468-2982.2008.01592.x
- Olesen J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. doi:10.1177/0333102417738202
- Goadsby PJ, Cohen AS, Matharu MS. Trigeminal autonomic cephalalgias: Diagnosis and treatment. Curr Neurol Neurosci Rep. 2007;7(2):117-125. doi:10.1007/s11910-007-0006-6
- Mitsikostas DD, Ashina M, Craven A, et al. European headache federation consensus on technical investigation for primary headache disorders. Journal of Headache and Pain. 2015;17(1):1-8. doi:10.1186/s10194-016-0596-y
- de Coo IF, Wilbrink LA, Haan J. Symptomatic Trigeminal Autonomic Cephalalgias. Current Pain and Headache Reports. 2015;19(8). doi:10.1007/s11916-015-0514-z
- Cittadini E, Matharu MS. Symptomatic trigeminal autonomic cephalalgias. Neurologist. 2009;15(6):305-312. doi:10.1097/NRL.0b013e3181ad8d67
- VanderPluym J. Cluster Headache: Special Considerations for Treatment of Female Patients of Reproductive Age and Pediatric Patients. Current Neurology and Neuroscience Reports. 2016;16(1):1-10. doi:10.1007/s11910-015-0610-9
- Cittadini E, Matharu MS, Goadsby PJ. Paroxysmal hemicrania: A prospective clinical study of 31 cases. Brain. 2008;131(4):1142-1155. doi:10.1093/brain/awn010
- Marmura MJ, Silberstein SD, Gupta M. Hemicrania continua: Who responds to indomethacin?. Cephalalgia. 2009;29(3):300-307. doi:10.1111/j.1468-2982.2008.01719.x
- Jürgens TP, Schulte LH, May A. Indomethacin-induced de novo headache in hemicrania continua-fighting fire with fire. Cephalalgia. 2013;33(14):1203-1205. doi:10.1177/0333102413490345
- Zhu S, McGeeney B. When Indomethacin Fails: Additional Treatment Options for “Indomethacin Responsive Headaches.” Current Pain and Headache Reports. 2015;19(4). doi:10.1007/s11916-015-0475-2
- Benoliel R, Sharav Y, Haviv Y, Almoznino G. Tic, Triggering, and Tearing: From CTN to SUNHA. Headache. 2017;57(6):997-1009. doi:10.1111/head.13040
- Rossi P, Tassorelli C, Allena M, Ferrante E, Lisotto C, Nappi G. Focus on therapy: hemicrania continua and new daily persistent headache. The journal of headache and pain. 2010;11(3):259-265. doi:10.1007/s10194-010-0194-3
- Rozen TD. How effective is melatonin as a preventive treatment for hemicrania continua? A clinic-based study. Headache. 2015;55(3):430-436. doi:10.1111/head.12489
- Belin AC, Ran C, Edvinsson L. Calcitonin gene-related peptide (CGRP) and cluster headache. Brain Sciences. 2020;10(1). doi:10.3390/brainsci10010030
- Khatami R, Tartarotti S, Siccoli MM, Bassetti CL, Sándor PS. Long-Term Efficacy of Sodium Oxybate in 4 Patients with Chronic Cluster Headache. 2011.
- Brandt RB, Doesborg PGG, Haan J, Ferrari MD, Fronczek R. Pharmacotherapy for Cluster Headache. CNS Drugs. 2020;34(2):171-184. doi:10.1007/s40263-019-00696-2
- Sewell RA, Halpern JH, Pope HG. Response of cluster headache to psilocybin and LSD. Neurology. 2006;66(12):1920-1922. doi:10.1212/01.wnl.0000219761.05466.43
- Am S, Onta TD. The New Eng Land Jour Nal of Medicine Treatment of Patients with Persistent Symptoms and a History of Lyme Disease. 2001;345. www.nejm.org
- Bartsch T, Falk D, Knudsen K, et al. Deep brain stimulation of the posterior hypothalamic area in intractable short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT). Cephalalgia. 2011;31(13):1405-1408. doi:10.1177/0333102411409070
- Lyons MK, Dodick DW, Evidente VGH. Responsiveness of short-lasting unilateral neuralgiform headache with conjunctival injection and tearing to hypothalamic deep brain stimulation: Case report. Journal of Neurosurgery. 2009;110(2):279-281. doi:10.3171/2008.4.17493
- Leone M, Franzini A, Proietti Cecchini A, Mea E, Broggi G, Bussone G. Deep Brain Stimulation in Trigeminal Autonomic Cephalalgias. Neurotherapeutics. 2010;7(2):220-228. doi:10.1016/j.nurt.2010.02.001
- Bartsch T, Paemeleire K, Goadsby PJ. Neurostimulation approaches to primary headache disorders. Current Opinion in Neurology. 2009;22(3):262-268. doi:10.1097/WCO.0b013e32832ae61e
- Schoenen J, di Clemente L, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache: A pilot study of efficacy and mode of action. Brain. 2005;128(4):940-947. doi:10.1093/brain/awh411
- Sebastian S, Schweitzer D, Tan L, Broadley SA. Role of trigeminal microvascular decompression in the treatment of SUNCT and SUNA. Current Pain and Headache Reports. 2013;17(5). doi:10.1007/s11916-013-0332-0
- Williams M, Bazina R, Tan L, Rice H, Broadley SA. Microvascular decompression of the trigeminal nerve in the treatment of SUNCT and SUNA. Journal of Neurology, Neurosurgery and Psychiatry. 2010;81(9):992-996. doi:10.1136/jnnp.2009.182824
- Marin J, Giffin N, Consiglio E, McClure C, Liebler E, Davies B. Non-invasive vagus nerve stimulation for treatment of cluster headache: Early UK clinical experience 11 Medical and Health Sciences 1103 Clinical Sciences. Journal of Headache and Pain. 2018;19(1). doi:10.1186/s10194-018-0936-1
- Matharu MS, Goadsby PJ. Trigeminal autonomic cephalgias. Neurology in Practice. 2002;72(2). doi:10.1177/2049463712456355
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013 Jul;33(9):629-808. doi: 10.1177/0333102413485658. PMID: 23771276.
- Alstadhaug KB, Ofte HK. Cluster headache. Tidsskr Nor Laegeforen. 2015 Aug 25;135(15):1361-4. English, Norwegian. doi: 10.4045/tidsskr.14.0824. PMID: 26315237.