
Article topic: Migraine
Author name: Yara Shatnawi
Keyword: chronic disease, pathophysiology, episodic migraine.
Overview:
Migraine is a chronic neurological disorder characterized by attacks of mild or severe headache and reversible neurological and systemic symptoms;1 some attacks preceded by aura and some without. The pathogenesis of migraines is not completely understood; it is thought to involve the trigeminal nerves and its additional axonal projections with the intracranial vascular system.2 Those with migraines may not receive the correct diagnosis, adequate treatment, or proper support.3 The economic and societal effect of migraine is substantial, it affects patients’ quality of life and reduces work efficiency, social activities, and family life.
Epidemiology:
Migraine is classified as the second most common neurological disorder; to which tension-type headache comes first, a female to male ratio of 3:1 and an estimated one year prevalence of 15% in the general population.5 the disease shows a peak prevalence in ages that range between 35-39 years with 75% of the general population suffering from a migraine attack before the age of 35 years.5,6 School-aged children on the other hand, show a one-year disease prevalence of 7%. 7 the disease leans towards remission in people aged 50 and above, an onset of a migraine attack among this age group usually leads to the suspension of secondary headache causes.5,8
Pathogenesis and etiology:
A migraine attack is divided into three phases:
- Premonitory
- Aura phase
- Headache phase.
- Premonitory phase
Migraine is accompanied by a non-painful symptomatology also known as the premonitory phase; this can also include aura; the symptoms can start hours to days prior to the attack and it can be recognized as a marker for an impending headache. The premonitory mostly included symptoms like, fatigue, yawning, changes in cognitive abilities, fluctuation in homeostasis, increased thirst, frequency of micturition and photophobia.8–19
Functional neuroimaging studies brought forth evidence regarding the association of the hypothalamus in the premonitory phase20, in a positron emission tomography study using the cerebral blood flow as a marker for patients with trinitrate-induced migraine attacks; it has been found that the activations were in:
- Posterolateral hypothalamus
- Midbrain tegmental area
- Dorsal pons
- Periaqueductal gray and multiple other cortical areas.
Functional magnetic resonance imaging was done during the interictal phase; defined as the time between migraine attacks. The results showed a strong association between the hypothalamus and areas related to pain transmission and autonomic function. This explains the autonomic symptoms experienced during the interictal and premonitory phase.21
There are two main theories that arised to explain the mechanism of action:
- There’s an increase in parasympathetic tone that lead to the activation of meningeal nociceptors and then subsequent vasodilation and release of local inflammatory mediators ( Figure 1).
- The regulation of nociceptive signals by neuropeptides from trigeminal nucleus caudalis to supratentorial structures required in processing pain.22,23
The parasympathetic system is enhanced and involved in the activation of meningeal nociceptors as evidenced by lacrimation, teary eyes, nasal congestion22
To elaborate more; multiple migraine triggers ( e.g. stress, sleep deprivation, food and etc) are involved in networks which project to preganglionic parasympathetic neurones located in the Superior Salivatory Nucleus ( SSN) this in turn leads to the release of neuropeptides in the postganglionic neurones located in the Sphenopalatine ganglion ( SPN) that innervates the blood vessels in the meninges.22

Figure 1: Activation of meningeal nociceptors by increased parasympathetic tone. BNST = bed nucleus of stria terminalis; LH = lateral hypothalamus; PAG = periaqueductal gray; Pir = piriform cortex; PVN = paraventricular hypothalamic nucleus; SPG = sphenopalatine ganglion; SSN = superior salivatory nucleus; TCC = trigeminal cervical complex; TG = trigeminal ganglion.24
- Migraine Aura
The changes that follow the onset of aura is thought to be explained by the cortical spreading depression which is a self-propagating wave of depolarization across the cerebral cortex, this causes cerebral hypoperfusion and ionic imbalance,25 those hemodynamic changes following cortical depression have been confirmed by neuroimaging techniques where patients who have migraine with aura showed changes that weren’t seen on images of patients who have migraine without aura.26 to elaborate more, the transient cortical depression ( Figure 2) is thought to result in the onset of aura by:
- Opening neuronal pannexin-1 channels,27
- Release of inflammatory mediators (e.g., nitric oxides and prostanoids) which are known for their vasodilatory effect28
- Vasodilatation of the intracranial arteries results in the stimulation and activation of trigeminal primary afferents that terminates in the perivascular space of intracranial arteries2 and function as transmitters of nociceptive impulses, the impulses are then relayed and processed into the cortical areas finally yielding a migraine attack.29,30

Figure 2: Cortical spreading depression. EEG = electroencephalogram; K+ = potassium.2
- Headache phase
The trigeminovascular system is believed to act as a substrate that will eventually yield a migraine attack by nocioceptive transmission.32 The trigemenal afferent fibers innervates the meninges and its respective blood vessels, in order for a migraine attack to initiate; first-order trigemenal neurones need to be sensitized and activated, as a consequence of activation, the neurones secretes vasoactive peptides (eg, substance P, calcitonin gene-related peptide) and stimulates local inflammatory reactions33 this whole process results in neurogenic inflammation of the meningeal vessels and propagation of trigeminal sensory fibers2 which in turn initiates the stimulation and activation of secondary and third-order neurons located in the brain stem and the thalamus respectively. Finally somatosensory and other cortical areas associated with pain sensation receive impulses from the nociceptors in order to manifest the migraine attack.2
Diagnosis and work up
The International Classification of Headache Disorders, 3rd edition (ICDH-3) provides a diagnostic criterion for migraine disorders, it mainly divides migraines into three subtypes:
- Migraine without aura.
- Migraine with aura.
- Chronic migraine.42
Table 1. ICHD-3 Diagnostic Criteria for Migraine without Aura
Table 2. ICHD-3 Diagnostic Criteria for Migraine with Aura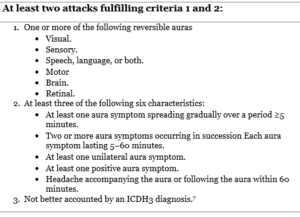
Visual auras account as the most common type of auras in the migraine with aura subtype; it most often presents as scintillations and scotoma; a less frequent form of auras are sensory disturbances followed by speech disturbances or aphasia. Sensory disturbances include the sensation of pins and needles affecting a significant or a minor area of the body like the tongue or face.
Aura symptoms can be single or multiple and usually follow the same order i.e visual, sensory then aphasia. The duration of most auras until the onset of headache is one hour (60 minutes), this may also occur in the absence of a following headache.7
Table 3. ICHD-3 Diagnostic Criteria for Chronic migraine

Differential diagnosis:
The differential diagnosis of headache includes other primary causes and secondary cause. Primary causes include tension-type headache. Secondary causes include subarachnoid haemorrhage, meningitis, encephalitis, carbon monoxide poising, etc.42
Treatment and Management:
The clinical management of patients with migraine is ideally managed and maintained by practitioners, if the patient’s diagnosis is challenging or the patient did not respond to any kind of treatment then referral to a specialist must be considered.7,43
- Acute and preventative methods with the use of non-pharmacological therapy as an adjunct to the already given medications.44–46
- Benefits were found in noninvasive neuromodulator devices, acupuncture and biobehavioral therapies.47–49
- Dietary approaches and physical therapy have shown no to little evidence regarding its benefits to therapy.7
There are acute and preventive pharmacological treatments for migraine, acute treatment is either specific (triptans and ergots) or non-specific (analgesics). preventive treatment reduces the frequency of attacks and improves quality of life. The plan to choose medications should be individualized that are specific to each patient, including the presence of associated symptoms like nausea or vomiting and the time to peak severity.
Acute treatment
Generally, headache phase especially early, is the best time to administer medications when trying to alleviate or remove the pain as a consequence of the attack.49
- Triptans, All triptans and triptan combination medications have a good evidence for effectiveness for acute treatment of migraine, in cases where oral triptans didn’t show any effect, other drugs of the same family can also provide pain relief .7 Currently there are seven oral triptans present (almotriptan, frovatriptan, sumatriptan, naratriptan, rizatriptan, zolmitriptan, and eletriptan), patients are advised to switch the type of oral Triptan when three attacks of migraines are treated with no success.50
- Analgesia including acetaminophen and certain nonsteroidal anti-inflammatory drugs (NSAIDs) include ibuprofen, diclonfenac, naproxen and acetyl-salicylic acid.51 Non-steroidal Anti-inflammatory Drugs (NSAIDS) are most used 52–54, this drug family has advantages over other drugs as it is of low cost and over-the-counter drug so easier accessibility.
When the treatment is only partially effective, clinicians can recommend a combination therapy including NSAIDs (e.g., naproxen sodium) with triptans.55 Subcutaneous triptans have shown better efficacy when compared with other triptans on the basis of patients who noted relief from pain after 2 hours from treatment. Thus, when presented with a patient who complains of inadequate treatment, subcutaneous triptans is the first choice.42,56 Triptans can cause vasoconstriction of arteries of coronary and limb arteries, therefore Triptans are contraindicated in patients with a history of stroke, myocardial infarction, coronary artery disease, hemiplegic migraine, uncontrolled hypertension, migraine with brainstem aura, and peripheral vascular disease.
Clinicians must be alert to the risk of medication overuse headache, which is a state where the patient expresses continuous headache or increase in the frequency of headache; a contribution of continuous medication overuse for migraines by patients who complain of headaches for 15 days per month.56 In such instances, medication withdrawal and initiation of preventative treatment is necessary.57–60
Recently, there has been enthusiasm regarding the small molecule calcitonin gene-related peptide CGRP receptor antagonists called gepants and 5-Hydorxytryptamine type 1-F (5-HT1F) receptor agonists called ditans, primarily used in the management of acute migraine.61 The Food and Drug Administration (FDA) has approved the use of the following oral ditans and geptans in the treatment of acute migraines: ubrogepant, lasmiditan and rimegepant. However due to its high costs and reduced availability, patients who are contraindicated or have shown ineffectiveness in the treatment modality with the use of NSAIDs or triptans are limited to the use of oral ditans and geptans. Moreover, Lismitdan have shown regression in driving and an insufficiency to assess ones driving expertise.61 As a consequence patient are recommended to avoid driving for at least 8 hours after ingestion.7,62 Thus, the broad use of Lismitdan can been limited by clinicians. Finally, guidelines are against the use of opioids and barbiturates due to its risk of dependency and its side effects.7
Preventive therapy of Migraine
The migraine prevention requires a comprehensive approach that include lifestyle modifications and trigger avoidance. Further interventions can include medications, nutraceuticals, behavioral therapies, and neurostimulation, where the aim is to reduce frequency of attacks and severity and duration.7
Generally, treatment is recommended for patients who have three headache per month that are ineffective to treatment and affect their societal life.63 unresponsive to acute therapy, accompanied by particularly bothersome symptoms (eg, brainstem aura, intractable vomiting), or when a patient is at risk of developing overuse of acute migraine medications.
The decision of which therapy to recommend is based on multiple factors include:
- The level of evidence that a specific therapy is effective.
- Its safety profile and cost.
- Patient comorbidities.
- Potential interactions with other therapies and if the patient tolerating the therapy or not.
Clinicians may use such information and advice the patients in order to reach to a mutual agreement about a realistic treatment plan. due to the restricted number of approved preventive medications, off-label use of medications is becoming more common.49
The most used preventive medications and nutraceuticals are topiramate, propranolol, nadolol, metoprolol, timolol, amitriptyline, gabapentin, candesartan, divalproex sodium, sodium valproate, flunarizine, pizotifen, venlafaxine, verapamil, lisinopril, coenzyme Q10, magnesium citrate, riboflavin, and feverfew. Chronic migraines have shown effectiveness with the use of toporimate and onabotulinimtoxinA (Botox).64–73
In addition, New preventative therapies have been introduced including monoclonal antibodies, they work by:
- Targeting a calcitonin gene-related peptide (CGRP) or its receptor (e.g., erenumab, galcanezumab, fremanezumab, and eptinezumab).
- As a prevention treatment of both episodic and chronic migraine attacks.64
- The drugs have rapid onset and few side effects, a common side effect include pain and erythema at the injection site.65
A 5 year open-lab extension study was done to study the long-term effect of erenumab, results showed that it continued to be safe in patients with diagnosed episodic migraines.74 The agents haven’t been compared to the above mentioned less expensive, more common and more accessible oral preventative medications. As a result, its recommended that the estimation and substitution of another medication can be considered after:
- Two to three months when treated with oral preventative therapy.
- Three to six months when treated with monoclonal antibodies that target CGRP or its receptors.
- Six to nine months when treated with Botox.75
Risk Factors
Several studies show that genetics play an essential role in the development of migraines where if one parent has migraine, chances of a child having migraine can rise up to 40%, if however, two parents have migraine chances increase to 75%.76 Other studies report an estimated 2.5% to 3% of people diagnosed with episodic migraines transitioned to chronic migraines each year.77 Risk factors for the development of chronic migraines include:
- Socioeconomic status, lifestyle factors and habits- obesity, low family socioeconomic status, female sex, daily caffeine intake and major life events.77
- Headache features and symptoms- increase in headache frequency, followed by headache persistent or frequent headaches associated with nausea and cutaneous allodynia.77
- Comorbid and concomitant diseases or conditions like depression, asthma, noncephalic pain, head and neck injury, snoring and insomnia.
- Pharmacological- acute medication overuse and acute migraine treatment efficacy.77
A few studies tested the outcomes of patients with chronic migraine when an intervention on modifiable risk factors was done, results showed a decrease in headache frequency and severity with some patients reporting remission from chronic migraine to episodic migraines.78
Prognosis
The prognosis of migraine is poorly studied and as a result research regarding this topic is still in its easily beginnings.79 Generally prognosis is divided into short term and long term. A study was done on 16,2576 individuals to assess the prevalence and prognosis by following up with the patients over the years, the results of the first wave (1-year) of follow up showed that:
- 84% of the individuals still had migraine.
- 10% showed 1-year clinical remission.
- 3% reported partial remission.
- 3% developed chronic migraines, the long-term outcomes weren’t reported.80
Few studies were done to assess the long-term outcomes in patients with migraines; a long duration follow up study was done in Sweden in 1955 to assess migraines in 9000 schoolchildren, a subgroup of those children [76 people] diagnosed with migraines were followed up after 40 years, around the age of 50, more than fifty percent of them still had migraine attacks.81
Another study was done on schoolchildren aged 7 years in Finish and were followed up 7 years later, of those children:
- 22% showed complete remission.
- 37% showed partial remission.
- 41% showed progression or persistence.82
Recent updates
A clinical trial was done on 1,072 people chronic migraineurs with a mean monthly migraine of 16.1 to assess the effect of epitnezumab. Adults were randomly divided and given IV epitnezumab 100 mg, epitnezumab 300 mg and placebo, this was done on day 1 and week 12. Results on week 12 showed significant decrease in mean monthly migraines in patients who took 100mg and 300 mg epitnezumab in comparison to placebo effects from day 1 through week 12.83
Another clinical trial was done to assess the effect of fremanezumab among people with episodic or chronic migraines by administering fremanezumab quarterly, or monthly over a period 12 weeks, responders to treatment were defined as those with reduction in monthly migraine days by at least 2 monthly migraine days in patients with episodic migraines and 4 monthly migraine days in patients with chronic migraines. Responders were identified and the results showed significant reduction in the monthly average number of migraine days by more than 50% in episodic migraines (EM: quarterly, 59,8%; monthly 63.7%) and chronic migraines (CM: quarterly, 52.8%; monthly, 59%). This can provide further understanding of the treatment gains in patients who responded to fremanezumab in clinical practice.83
References
- Dodick DW. Migraine. The Lancet. 2018;391(10127):1315-1330. doi:10.1016/S0140-6736(18)30478-1
- Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system—40 years and counting. The Lancet Neurology. 2019;18(8):795-804. doi:10.1016/S1474-4422(19)30185-1
- Burch R, Rizzoli P, Loder E. The Prevalence and Impact of Migraine and Severe Headache in the United States: Figures and Trends From Government Health Studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
- Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
- Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170-1178. doi:10.1111/j.1468-2982.2008.01666.x
- Wöber C, Wöber-Bingöl Ç, Uluduz D, et al. Undifferentiated headache: broadening the approach to headache in children and adolescents, with supporting evidence from a nationwide school-based cross-sectional survey in Turkey. Journal of Headache and Pain. 2018;19(1). doi:10.1186/s10194-018-0847-1
- Steiner TJ, Jensen R, Katsarava Z, et al. Aids to management of headache disorders in primary care (2nd edition): On behalf of the European Headache Federation and Lifting the Burden: The Global Campaign against Headache. Journal of Headache and Pain. 2019;20(1). doi:10.1186/s10194-018-0899-2
- Giffin NJ, Ruggiero ; L, Lipton ; R B, et al. Premonitory Symptoms in Migraine An Electronic Diary Study.; 2003.
- Kelman L. The Premonitory Symptoms (Prodrome): A Tertiary Care Study of 893 Migraineurs.
- Schoonman GG, Evers DJ, Terwindt GM, van Dijk JG, Ferrari MD. The prevalence of premonitory symptoms in migraine: A questionnaire study in 461 patients. Cephalalgia. 2006;26(10):1209-1213. doi:10.1111/j.1468-2982.2006.01195.x
- Quintela E, Castillo J, Muñoz P, Pascual J. Premonitory and resolution symptoms in migraine: A prospective study in 100 unselected patients. Cephalalgia. 2006;26(9):1051-1060. doi:10.1111/j.1468-2982.2006.01157.x
- Karsan N, Prabhakar P, Goadsby PJ. Characterising the premonitory stage of migraine in children: a clinic-based study of 100 patients in a specialist headache service. Journal of Headache and Pain. 2016;17(1). doi:10.1186/s10194-016-0689-7
- Cuvellier JC, Mars A, Vallée L. The prevalence of premonitory symptoms in paediatric migraine: A questionnaire study in 103 children and adolescents. Cephalalgia. 2009;29(11):1197-1201. doi:10.1111/j.1468-2982.2009.01854.x
- Amery WK, Waelkens J, Vandenbergh V. Original Articles Migraine Warnings. Vol 26.; 1986.
- Waelkens J. Warning symptoms in migraine: characteristics and therapeutic implications.
- Rasmussen BK, Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12:221-229.
- Laurell K, Artto V, Bendtsen L, et al. Premonitory symptoms in migraine: A cross-sectional study in 2714 persons. Cephalalgia. 2016;36(10):951-959. doi:10.1177/0333102415620251
- Rusinkiewicz WI, Phillips A. 10 Card WI. Rational Justification for Therapeutic Decisions. Vol 281. Oxford University Press; 1980.
- Russell MB, Rasmussen BK, Fenger K, Olesen J. Migraine without Aura and Migraine with Aura Are Distinct Clinical Entities: A Study of Four Hundred and Eighty-Four Male and Female Migraineurs from the General Population. Cephalalgia 1996;16(4): 239–245. Doi:10.1046/j.1468-2982.1996.1604239.x. .
- Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137(1):232-241. doi:10.1093/brain/awt320
- Moulton EA, Becerra L, Johnson A, Burstein R, Borsook D. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS ONE. 2014;9(4). doi:10.1371/journal.pone.0095508
- Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. In: Journal of Comparative Neurology. Vol 493. ; 2005:9-14. doi:10.1002/cne.20688
- Noseda R, Kainz V, Borsook D, Burstein R. Neurochemical pathways that converge on thalamic trigeminovascular neurons: Potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS ONE. 2014;9(8). doi:10.1371/journal.pone.0103929
- Dodick DW. A Phase-by-Phase Review of Migraine Pathophysiology. Published online 2018. https://headachejournal.onlinelibrary.wiley.com/cms/asset/d1f9782e-19ea-4187-8f93-1d4ca8f1c27f/head13300-fig-0001-m.jpg
- Sanchez de M. Perfusion Weighted Imaging during Migraine: Spontaneous Visual Aura and Headache.
- Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123). doi:10.1126/science.1231897
- Ashina M, Hansen JM, Á Dunga BO, Olesen J. Human models of migraine-short-Term pain for long-Term gain. Nature Reviews Neurology. 2017;13(12):713-724. doi:10.1038/nrneurol.2017.137
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Published online 2002. doi:10.1038/nm0202-136
- Dodick DW. A Phase‐by‐Phase Review of Migraine Pathophysiology. Headache: The Journal of Head and Face Pain. 2018;58:4-16. doi:10.1111/HEAD.13300
- Dodick DW. a phase-by-phase review of migraine pathophysiology. Published online 2018. https://headachejournal.onlinelibrary.wiley.com/cms/asset/d1f9782e-19ea-4187-8f93-1d4ca8f1c27f/head13300-fig-0001-m.jpg
- Moskowitz MA. The Neurobiology of Vascular Head Pain. Vol 16.; 1984.
- Brust JCM (John CM). Current Diagnosis & Treatment. Neurology.
- Al-Karagholi MAM, Hansen JM, Guo S, Olesen J, Ashina M. Opening of ATP-sensitive potassium channels causes migraine attacks: A new target for the treatment of migraine. Brain. 2019;142(9):2644-2654. doi:10.1093/brain/awz199
- Negro A, Delaruelle Z, Ivanova TA, et al. Headache and pregnancy: a systematic review. Journal of Headache and Pain. 2017;18(1). doi:10.1186/s10194-017-0816-0
- Reuter U, McClure C, Liebler E, Pozo-Rosich P. Non-invasive neuromodulation for migraine and cluster headache: A systematic review of clinical trials. Journal of Neurology, Neurosurgery and Psychiatry. 2019;90(7):796-804. doi:10.1136/jnnp-2018-320113
- Sharpe L, Dudeney J, Williams AC de C, et al. Psychological therapies for the prevention of migraine in adults. Cochrane Database of Systematic Reviews. 2019;2019(7). doi:10.1002/14651858.CD012295.pub2
- Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database of Systematic Reviews. 2016;2016(6). doi:10.1002/14651858.CD001218.pub3
- Luedtke K, Allers A, Schulte LH, May A. Efficacy of interventions used by physiotherapists for patients with headache and migraine – Systematic review and meta-analysis. Cephalalgia. 2015;36(5):474-492. doi:10.1177/0333102415597889
- Chaibi A, Benth J, Tuchin PJ, Russell MB. Chiropractic spinal manipulative therapy for migraine: a three-armed, single-blinded, placebo, randomized controlled trial. European Journal of Neurology. 2017;24(1):143-153. doi:10.1111/ene.13166
- AtlAs of Headache Disorders and Resources in the World 2011.; 2011. www.who.int
- Law S, Derry S, Moore RA. Sumatriptan plus naproxen for the treatment of acute migraine attacks in adults. Cochrane Database of Systematic Reviews. 2016;2016(4). doi:10.1002/14651858.CD008541.pub3
- Dahlöf CGH. Infrequent or non-response to oral sumatriptan does not predict response to other triptans – Review of four trials. Cephalalgia. 2006;26(2):98-106. doi:10.1111/j.1468-2982.2005.01010.x
- Rabbie R, Derry S, Moore RA, McQuay HJ. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. In: Moore M, ed. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2010. doi:10.1002/14651858.CD008039.pub2
- Saguil A, Herness J. Aspirin with or without an antiemetic for acute migraine headaches in adults. American Family Physician. 2014;89(3):176-177. doi:10.1002/14651858.cd008041.pub2
- Derry S, Rabbie R, Moore RA. Diclofenac with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews. 2013;2017(9). doi:10.1002/14651858.CD008783.pub3
- Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults – overview of Cochrane reviews. Cochrane Database of Systematic Reviews. 2014;2014(5). doi:10.1002/14651858.CD009108.pub2
- Diener HC, Dodick D, Evers S, et al. Pathophysiology, prevention, and treatment of medication overuse headache. The Lancet Neurology. 2019;18(9):891-902. doi:10.1016/S1474-4422(19)30146-2
- Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the Treatment of Migraine. New England Journal of Medicine. 2019;381(23):2230-2241. doi:10.1056/nejmoa1813049
- Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: The achieve ii randomized clinical trial. JAMA – Journal of the American Medical Association. 2019;322(19):1887-1898. doi:10.1001/jama.2019.16711
- Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. The Lancet. 2019;394(10200):737-745. doi:10.1016/S0140-6736(19)31606-X
- Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894-1904. doi:10.1093/brain/awz134
- Pearlman EM, Wilbraham D, Dennehy EB, et al. Effects of lasmiditan on simulated driving performance: Results of two randomized, blinded, crossover studies with placebo and active controls. Human Psychopharmacology. 2020;35(5). doi:10.1002/hup.2732
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The american headache society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3-20. doi:10.1111/head.12499
- The American Headache Society Position Statement On Integrating New Migraine Treatments Into Clinical Practice. Headache. 2019;59(1):1-18. doi:10.1111/head.13456
- Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. The Lancet. 2019;394(10210):1765-1774. doi:10.1016/S0140-6736(19)32504-8
- Ashina M, Goadsby PJ, Reuter U, et al. Long-term safety and tolerability of erenumab: Three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39(11):1455-1464. doi:10.1177/0333102419854082
- Goadsby PJ, Reuter U, Hallström Y, et al. A Controlled Trial of Erenumab for Episodic Migraine. New England Journal of Medicine. 2017;377(22):2123-2132. doi:10.1056/nejmoa1705848
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. The Lancet Neurology. 2017;16(6):425-434. doi:10.1016/S1474-4422(17)30083-2
- Dodick DW, Silberstein SD, Bigal ME, et al. Effect of Fremanezumab compared with placebo for prevention of episodic migraine a randomized clinical trial. JAMA – Journal of the American Medical Association. 2018;319(19):1999-2008. doi:10.1001/jama.2018.4853
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the Preventive Treatment of Chronic Migraine. New England Journal of Medicine. 2017;377(22):2113-2122. doi:10.1056/nejmoa1709038
- Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):E2211-E2221. doi:10.1212/WNL.0000000000006640
- Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241-254. doi:10.1177/0333102420905132
- Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94(13):E1365-E1377. doi:10.1212/WNL.0000000000009169
- Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE-1 randomized clinical trial. JAMA Neurology. 2018;75(9):1080-1088. doi:10.1001/jamaneurol.2018.1212
- Ashina M. Migraine. Ropper AH, ed. New England Journal of Medicine. 2020;383(19):1866-1876. doi:10.1056/NEJMra1915327
- MacGregor EA. Migraine. Annals of internal medicine. 2017;166(7):ITC49-ITC64. doi:10.7326/AITC201704040
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache. 2008;48(8):1157-1168. doi:10.1111/j.1526-4610.2008.01217.x
- Burch RC, Buse DC, Lipton RB. Migraine: Epidemiology, Burden, and Comorbidity. Neurologic Clinics. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
- Bigal ME, Lipton RB. The Prognosis of Migraine.
- Lipton RB, Bigal ; M E, Diamond ; M, Freitag ; F, Reed ML, Stewart WF. Migraine Prevalence, Disease Burden, and the Need for Preventive Therapy.; 2007.
- bille1997.
- Sillanpää M, Anttila P. Original Articles Increasing Prevalence of Headache in 7-Year-Old Schoolchildren.
- Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94(13):E1365-E1377. doi:10.1212/WNL.0000000000009169
- Silberstein SD, Cohen JM, Yang R, et al. Treatment benefit among migraine patients taking fremanezumab: results from a post hoc responder analysis of two placebo-controlled trials. Journal of Headache and Pain. 2021;22(1). doi:10.1186/s10194-020-01212-4







The article is very informative, well-written, and straight to the point. Great job, keep us updated with your work