Article topic: Guillain barré syndrome
Name of the author: Abdallah Said Al-hallaq
Keywords: GBS, Guillain barré syndrome, Plasma exchange, SARS COV-2
History of Guillain-Barré syndrome
The history of Guillain-Barré syndrome (GBS) is almost related to the discovery of the peripheral nervous system. Up to the second half of the 19th century, any injury to the peripheral nervous system had not yet emerged as a possible cause of palsy. In 1928, there was an epidemic occurring at an infirmary in Paris, manifestations included unpleasant tingling sensations associated with severe pain and weakness of the lower limbs. During the second half of the 19th century, many similar cases were reported. This epidemic was undoubtedly more related to a toxic phenomenon than to inflammation.
The final step in the description of GBS was the publication of an article in 1916 signed by Guillain-Barre and Strohl with the title of “Radiculoneuritis syndrome with hyperalbuminosis of cerebrospinal fluid without a cellular reaction”1.
The term GBS was introduced for the first time in Paris in 1927 by Draganescu and Claudian who had described that a radiculoneuritis that occurs after staphylococcal osteomyelitis is GBS.
Overview and epidemiology
Guillain-Barré syndrome (GBS) is a collection of acute autoimmune-related disorders of peripheral nerves with a monophasic course, and the progressive phase of GBS should be less than four weeks. there are many clinical variants of GBS that have been documented in the medical literature 2, the most common variants are acute inflammatory polyradiculoneuropathy (AIDP) and acute motor axonal neuropathy (AMAN).
The disorder was first described as a benign form of limb weakness that is associated with full recovery, but now we recognize GBS as a prolonged, disabling neuromuscular disorder associated with respiratory difficulties in nearly a third of patients3.
Its incidence ranges between 0.40 and 3.25 cases per 100,000 persons, depending on geographic location. Despite the fact that its rare, Guillain-Barre syndrome (GBS) has a lot of major effects on the health care system4, In different age groups, the annual average rate of hospitalizations in the US related to GBS increases with age, being 1.5 cases per 100,000 population in persons younger than 15 years and peaking at 8.6 cases per 100,000 population in people aged 70-79 years5.
Differences in the incidence of GBS in different populations could reflect variations in genetic susceptibility or in the exposure to causative pathogens like Campylobacter jejuni been the most widely reported infection: it has been found in 25–50% of adult patients, and is more frequent in Asian countries6.
GBS is slightly more frequent in males than in females, it has a male-to-female ratio of 1.5:1 and this male preponderance is seen in older patients especially. However, there is Swedish epidemiologic study has reported that GBS rates decrease during pregnancy and increase in the months immediately following delivery7.
GBS occurs in any age group, the syndrome can occur at any time between infancy and old age. It has been reported in the United States that the syndrome’s age distribution seems to be bimodal which means that it comes with 2 peaks the first peak in young adulthood (ages 15-35 y) and a second higher one in middle-aged and elderly persons (ages 50-75 y)8. Infants appear to have the lowest risk of developing GBS.
Guillain-Barré Syndrome and Criteria for Diagnosis
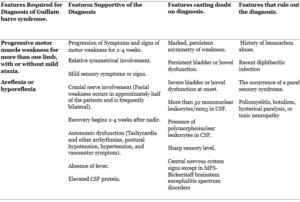
Diagnostic criteria for Guillain-Barré syndrome (GBS) took place in 1978 upon the request of the National Institute of Neurological and Communicative Disorders and Stroke NINCDS which now is called NIND, summarized in TABLE (1). This criterion is related to the swine of flu vaccine incident of 1976-19779.
Progressive motor muscle weakness for more than one limb and hypo/areflexia are the two clinical features required for the diagnosis of paralytic GBS.
In general, the diagnosis of GBS is typical and almost straight forward and the differentials are narrow but there are some patients with atypical presentation. Typically, the patient presents 2-4 weeks after a mild respiratory or gastrointestinal infection with progressive monophasic generalized and symmetric weakness, paresthesia, numbness these are the most common complaints.10 The symptoms at the time of onset of classic GBS are pain, paresthesia, numbness, and rapidly progressive bilateral limb weakness3,11.
TABLE (1): National Institute of Neurological Disorders and Stroke Diagnostic Criteria for Guillain-Barré Syndrome12
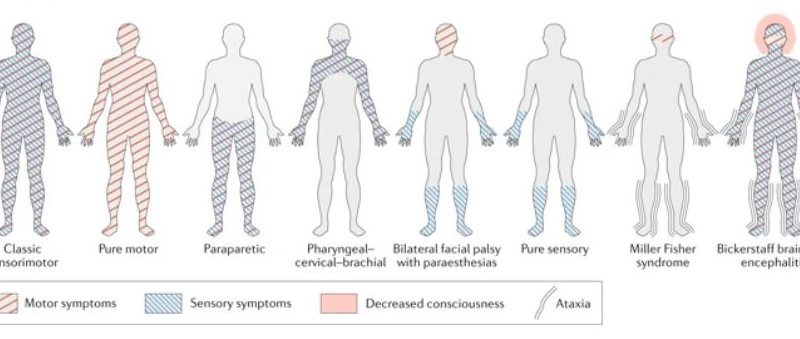
GBS variants
There are different clinical variants that are based basically on
- The types of nerve fibers involved (motor, sensory, sensory, and motor, cranial or autonomic).
- The predominant mode of fiber injury (demyelinating versus axonal).
- The presence of an alteration in consciousness.
Acute inflammatory demyelinating polyneuropathy (AIDP) is the most frequent variant of GBS in developed countries, AIDP is also the predominant subtype around 60–80% of patients who are in North America and Europe13, However, in Asia, Central and South America the axonal variants of GBS like acute motor axonopathy (AMAN) and acute motor-sensory axonopathy (AMSAN) represent 30% to 47% of cases.
The axonal motor variants of GBS termed acute motor axonal neuropathy (AMAN), is called Chinese paralytic illness because it was reported in 1993 from Northern China14. And not very late later that an acute motor and sensory axonal neuropathy (AMSAN) was discovered15. Both AMAN and AMSAN are usually associated with clostridium Jejuni infection which is alone is considered a poor prognostic factor16. Patients with this AMAN variant present with symmetric proximal and distal weakness without sensory abnormalities following C. jejuni enteritis and could have normal or brisk tendon reflexes. Moreover, patients with AMSAN have a poor prognosis because of severe involvement of sensory and motor nerve fibers and more likelihood of autonomic involvement.
There is another variant of GBS is Miller Fisher Syndrome (MFS) and it presents opthalmoplegia (first presenting symptom) with ataxia and areflexia but without any weakness17. Miller fisher syndrome patients have at least 2 of these symptoms and have in support an elevated CSF protein and characteristic autoantibody.
MFS is more common in Eastern Asia accounting for up to 25% of Japanese cases, and it’s thought to represent 5 to 10% of GBS cases In Western countries18.
Bickerstaff’s brain stem encephalitis (BBE) and is a variant of MFS characterized by alteration in consciousness, paradoxical hyperreflexia, ataxia, and ophthalmoparesis19, it represents a variant of that is with antecedent infection (92%), elevated CSF protein (59%), and anti-GQ1b antibody (66%)20.
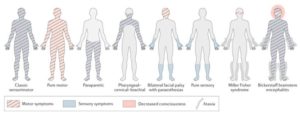
Figure (1) Pattern of symptoms invariants of Guillain–Barré syndrome21
Clinical presentation and complications
The features which allow a diagnosis for GBS include clinical, laboratory, and electrodiagnostic criteria. The presence of preceding events is frequent and could be seen, but they are not essential to the diagnosis, so the most commonly preceding events are usually viral infections but there are others like preceding surgery, inoculations, and Mycoplasma infections is also could be seen.
- Muscle Weakness
In a typical presentation of Guillain-Barré syndrome, rapidly progressive bilateral weakness is usually the key symptom presenting in most patients, The weakness usually starts in the distal lower extremities and then progresses upwards over a period of hours to days (ascending symmetrical weakness), until the arms and facial muscles also are affected, which leads to bulbar weakness and respiratory difficulties22.
The muscle weakness could start in the arms first (the descending type), or simultaneously in the arms and legs, and in some other forms of the disease, the weakness could only affect the cranial nerves, leading to facial, oculomotor, or bulbar weakness23. There is a small number of patients present with paraparesis, which unfortunately may remain during the course of the disease24.
The weakness reaches its peak after 2 to 4 weeks from symptom onset and should be symmetrical, if it is asymmetrical this calls the diagnosis of GBS into question.
2. Cranial nerve
Neurological examination is significant in those patients, as we could see cranial nerves signs like most frequently observed is Facial weakness (VII), followed by signs associated with cranial nerves VI, III, XII, V, IX, and X25. these patients could present with:
- Ptosis from cranial nerve III (oculomotor) palsy.
- Ophthalmoparesis may be observed in up to 25% of patients with GBS26
- Facial droop
- Dysphagia, dysarthria, and findings associated with disorders of the eye.
- In severe cases, tonic pupils have been observed.
3. Reflexes
Areflexia or hyporeflexia is one of the two clinical features required for the diagnosis of GBS and it is unusual to see normal reflexes or hyperreflexia throughout the course of GBS.27
Hyperreflexia could indicate that there is functional corticospinal tract involvement in patients with a GBS variant, and there is a study that showed 48% of Chinese and 33% of Japanese patients with AMAN showed hyperreflexia in the recovery phase14. Bickerstaff brainstem encephalitis might be present with hyperreflexia that associated with external ophthalmoplegia and ataxia.
Reflexes typically are decreased or absent but initially might be closed to normal in pure motor and axonal forms or sometimes even hyper-reflexes.
4. Sensory symptoms and pain
Most patients note paresthesia, numbness, or similar sensory changes, although prominent sensory signs are atypical (except in sensory variants).
The most common initial symptom is Acroparesthesia28, progressing upward but generally not extending beyond the wrists or ankles and it could be accompanied by Loss of vibration, proprioception, touch, and pain distally may be present.
Moderate to severe neuropathic or radicular pain is a common that is seen in all variants of GBS including MFS29, Persistent pain has been reported in the 2 weeks preceding weakness in 36% of patients while 66% of reported pain in the acute phase and 38% reported pain after 1 year29.
Pain in Guillain-Barre´ syndrome (GBS) may be pronounced and is often overlooked. Muscle pain or radicular pain, often but not always in the spinal region, the pain is often described as aching or throbbing in nature.
5. Autonomic changes
In patients with GBS a dysfunction in the sympathetic and parasympathetic systems and could present with30:
- Most commonly of sinus tachycardia, but patients may experience bradycardia
- Facial flushing
- Cardiac arrhythmias
- Paroxysmal hypertension
- Orthostatic hypotension
- Anhidrosis and/or diaphoresis
5% of cases experience bladder (urinary retention) and gastrointestinal (constipation, ileus, gastric distension, diarrhea, fecal incontinence) dysfunction and these symptoms are unusual at the onset of the disease but commonly develop at the nadir of the disease26.
Also, GBS patients can develop autonomic dysfunction, with usually GI manifestations diarrhea or constipation in 15% of patients also part of them develop hyponatremia (15%) with a smaller number of them develop a syndrome of inappropriate antidiuretic hormone secretion (5%), bradycardia (5%), and urinary retention (4%)31.
As a result of autonomic nervous system involvement there could be on clinical exam32:
- Cardiac arrhythmias either tachycardia or bradycardia, and tachypnea could be a sign of ongoing dyspnea and progressive respiratory failure.
- absent bowel sounds indicate paralytic ileus.
- Variations of hypertension and hypotension also could be observed (Blood pressure lability).
- poor inspiratory effort or diminished breath sounds.
- Suprapubic tenderness or fullness may be suggestive of urinary retention.
6. Respiratory involvement
Given that up to 30% of GBS cases progress to respiratory failure26, neuromuscular respiratory failure is the most thing that worries the clinicians, it could occur after the involvement of the lower cranial nerves (glossopharyngeal, vagus, and hypoglossal nerves) or after the involvement of the nerves of the muscles of respiration may lead to the need for artificial ventilation and it’s a life-threatening complication33. The weakness of the diaphragm is thought to be caused by phrenic nerve demyelination34
They present with:
- Dyspnea on exertion
- Shortness of breath
- Difficulty swallowing
- Slurred speech
Approximately 25% of patients develop respiratory insufficiency requiring artificial ventilation35, that why good supportive care is the most important element of management.
Work up and diagnosis (Approach considerations)
In most cases, we consider Guillain-Barre syndrome (GBS) a clinical diagnosis because of that the diagnosis could be done at the bedside12.
Peripheral Neuropathy Workup
These basic Peripheral Neuropathy Workup
studies may include:
- Thyroid function test
- B12, Folate, Hba1c
- Rheumatology profiles
- Erythrocyte sedimentation rate (ESR)
- Check for SIADH: hypokalemia and hyponatremia.
Biochemical Screening
Biochemical screening includes Electrolyte levels, Liver function tests (LFTs), Creatine phosphokinase (CPK) levels. In a third of GBS patients, LFT results are elevated36.
In some cases, we order a pregnancy test because in rare cases a high suspicion for an early diagnosis and prompt intensive supportive care in a GBS-complicated pregnancy will likely improve the prognosis for both mother and fetus37.
Lumbar puncture
it is common to perform CSF analysis to support your diagnosis, Lumbar puncture and cerebrospinal fluid analysis show a classic pattern of albuminocytologic dissociation which means that CSF typically reveals high protein levels with a normal white blood cell count but this pattern is present only in approximately 80% of patients after 2 weeks following the symptoms onset1.
Nerve conduction studies
Nerve conduction studies and the Electromyography are far more useful for diagnosis and helpful in diagnosing GBS and differentiate it from another disease that mimics it. Decreased conduction velocities or Conduction block are indicators of the demyelination process and there are specific tests for proximal nerve involvement that can be seen early in the disease including the recording of the F-waves and abnormalities like dispersion or dropout in F-wave signal38, Prolongation or absence of the F-waves latencies is a common finding during the first week
Signs of demyelination on motor nerve conductions in AIDP:
- Prolongation or absence of the F-waves latencies
- Prolonged distal motor latencies
- Conduction blocks at non-compressible sites.
In an axonal variant of the disease there will be decreased compound muscle action potential (CMAP) amplitudes in AMAN and decreased CMAP and sensory nerve action potential (SNAP) amplitudes in AMSAN.
Serologic Studies
Check for antibodies to the following infectious agents should be considered:
- C jejuni
- Cytomegalovirus (CMV)
- Epstein-Barr virus (EBV)
- Herpes simplex virus (HSV)
- HIV
- Mycoplasma pneumonia
The spectrum of GBS subtypes and serum antiganglioside antibodies
- Acute inflammatory demyelinating polyradiculoneuropathy (AIDP): Unknown antibodies39
- Acute motor (and sensory) axonal neuropathy (AMAN or AMSAN): GM1, GM1b, GD1a, GalNAc-GD1a40
- MFS: GD3, GT1a, GQ1b41.
Pulmonary function test (PFT):
Forced vital capacity (FVC) is very helpful in determining the best management of Guillain-Barre syndrome (GBS)42, for those Patients who have FVC of < 15-20 mL/kg, maximum inspiratory pressure of < 30 cm water, or a maximum expiratory pressure < than 40 cm water generally progress to require prophylactic intubation and mechanical ventilation and need fast intervention. Respiratory assistance should also be considered when there is a decrease in oxygen saturation (arterial partial pressure of oxygen [PO2] < 70 mm Hg)42.
For difficult bedside patients, we perform Negative inspiratory force (NIF) which is a relatively easy bedside test to measure respiratory muscle function and can be performed easily every half hour to hour43.
Magnetic resonance imaging
Brain or spine MRI could show enhancement of the spinal roots of cranial nerves in patients with GBS44 and might be useful to rule out another differential diagnosis.
Pathogenesis and etiology
GBS tends to be a postinfectious and immune-mediated disease that targets peripheral nerves and there is about two-thirds of the patients had symptoms of an infection in the 3 weeks before the onset of weakness.
Previous infections are reported in up to 70% of patients with Guillain-Barre Syndrome so it is basically it is a postinfectious, immune-mediated disease so Cellular and humoral immune mechanisms probably play a role in its development45.
Many patients report an infectious illness week prior to the onset of GBS, many of the identified infectious agents are thought to be inducing the production of antibodies that cross-react with specific gangliosides and glycolipids, such as GM1 and GD1b, that are distributed throughout the myelin sheath in the peripheral nervous system11.
The fact that many cases of Guillain-Barré syndrome begin after a viral or bacterial infection suggests that there are certain characteristics of some viruses and bacteria that may activate the immune system inappropriately in a way or another.
Many infectious micro-organisms are linked to the onset of GBS, and Campylobacter jejuni is the most commonly associated specifically (serotype O:19), with the onset of axonal injury and Wallerian degeneration that leads to irreversible forms of GBS46.
Research studies have also shown other triggers for GBS like Cytomegalovirus, Epstein Bar virus, Haemophilus influenza, and COVID-19.
In December 2019, the novel COVID-19 has been discovered in Wuhan (China), the pandemic disease had a number of neurological complications that have been reported. there was a confirmed GBS case however new studies show that Neuroscientists have found no significant association between COVID-19 and Guillain-Barré syndrome, their analysis shows that SARS-CoV-2 contains no additional immunogenic material known or proven to drive GBS47.
There is a Concern that COVID-19 vaccination might cause GBS in any significant numbers are therefore almost certainly unfounded, so far there are no cases found related to the vaccine but with time we will be sure about it47.
An epidemiological study in 1976, has shown a large increase in the incidence of GBS cases after influenza A vaccines in that year. However, recent studies have shown that the risk of acquiring GBS is only slightly higher after the vaccination and that there is a larger benefit in reducing the occurrence of GBS after vaccination48.
There are other triggers such as Trauma, surgery, pregnancy these all could be possible triggers.
Treatment and therapeutic approach
GBS patients are at high risk of developing many complications, some of them may lead to death so because of that close monitoring is crucial to predict and prevent potential complications so the monitoring of respiratory, cardiac, and hemodynamic function is needed to prevent or manage complications.
The treatment of GBS needs a many-sided approach consisting of general medical care and immunological treatment.
General medical care
Good supportive care is crucial in the treatment of patients with GBS49, As of monitoring the respiratory, cardiac and hemodynamic function because this is our main concern at the beginning, Prophylaxis for deep vein thrombosis is generally accepted, although it has never been subjected to controlled trial, we also check of the possible bladder and bowel dysfunction and we do Pain management using opioids or non-steroidal anti-inflammatory drugs29, psychosocial care is also reported to be important.
Prehospital admission should be carefully done with (ABCs) airway, breathing, and circulation and should be immediately admitted to the hospital for close monitoring because the symptoms could progress rapidly50. Atropine is recommended for symptomatic bradycardia.
acute neuromuscular paralysis and its accompanying complications it’s what worry us so patients need to be monitored closely in ICUs by physicians who are experienced and know how to deal with it.
In ICU we should focus on:
- Respiratory therapy with Cardiac monitoring
- Monitoring for infectious complications (eg, pneumonia, urinary tract infections, septicemia.
There is a low threshold for intubation and if (FVC) falls to be below 1200 ml or 15 ml/kg and/or negative inspiratory force drops below 20 cm H2O, then intubation and respiratory support should be considered51.
We should in ICU keep monitoring of respiratory function by frequent measurement of vital capacity and other clinical parameters, such as respiratory depth, respiratory frequency and cough strength35
Indications for ICU admission52:
- All patients come with labile dysautonomia.
- All patients with a forced vital capacity of less than 20 mL/kg.
- Patients with severe bulbar palsy.
- All patients exhibiting any clinical signs of respiratory compromise to any degree.
- Labile blood pressure.
- Cardiac arrhythmia.
Pain management
Medications for pain may be required in inpatient or outpatient settings. We start with nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen, with narcotic agents added if needed, it is usually recommended50.
Opioids could be used for short-term treatment of pain but should be avoided for long-term pain management53.
Immunological treatment
Immunomodulating therapy, mainly intravenous immunoglobulin (IVIg) therapy and plasma exchange (PE), have been proved to be beneficial in improving outcome54, either IVIg or Plasma Exchange should be started as soon as we can before any irreversible damage has taken place and make the situation even worse.
Immunotherapy is started usually for GBS patients if they are not able to walk 10 m unaided (GBS Disability Scale score ≥3)55.
As IVIg is generally more widely available than plasma exchange because of its widespread availability and its good tolerability for the patients and is given at a dose of 0.4 g/kg daily for 5 consecutive days (or 2 g/kg over 3-5 days)56, It’s effective as a full course of five plasma exchange sessions applied over 2 weeks57.
Plasma exchange helps in taking off autoantibodies, immune complexes, and cytotoxic constituents from serum and helps in decreasing the recovery time to half, A review of 6 randomized, controlled trials has been made involving 649 participants have found that PE helped speeding up the recovery time from GBS without causing any harm, apart from a slightly increased risk of relapse58. the standard dose of plasma exchange is to exchange 200 mL/kg to 250 mL/kg in four to five sessions over 7 to 14 days59.
Combined Plasma Exchange and IVIg have been shown to be no better than Plasma Exchange or IVIg alone, studies of given plasma exchange followed by IVIg has been of no benefit13.
Immunotherapy for pregnant women has not been studied well yet and safety for use during pregnancy has not been established and immunotherapy for children with GBS has not been studied yet with randomized, well-controlled studies that could be taken, but it is a standard aspect of treatment in this age group13.
Prognosis and outcome
The outcomes of GBS are highly variable but their accurate prediction is critical to enable the clinicians to give supportive care and treatment to the patients’ needs and to inform patients and their relatives about the exact clinical course of the disease to be well prepared for anything.
Predicators for the need for ventilation
There have been three studies that have been performed to predict the probability of respiratory insufficiency risk in GBS patients. The first one was a French study including 722 patients and found that time from onset to the admission of fewer than 7 days, inability to stand, inability to cough and the inability to lift the elbows or head from the bed, and increased liver enzyme levels were somehow predictors of an increased probability of the need for artificial ventilation for them60.
A second study was a French study has found that if there were a peroneal nerve conduction block and low vital capacity it is mostly correlated with a high risk of respiratory failure61.
The third study has taken place in the Netherlands and they used data from a derivation cohort of 397 patients with GBS to identify clinical predictors of mechanical ventilation, which then were validated in an independent cohort of 191 patients with GBS61. The results of this study led to the development of what it called the Erasmus GBS Respiratory Insufficiency Score (EGRIS)62. The EGRIS is an accurate prediction model score that could be used in the emergency department to predict the probability of respiratory insufficiency during the first week after admission for GBS62. The score contains the following parameters: weakness severity (expressed as the MRC sum score), the number of days between the onset of weakness and admission, and facial and/or bulbar weakness. If the patient has a high probability of developing Respiratory insufficiency, we would rather admit the patient to the ICU rather than to a general neurology ward.
Predictors of poor long-term outcomes
Poor outcome is defined in almost all studies as a GBS Disability Scale score of 3 or more after either 6 months or 12 months63, also diarrhea preceding GBS onset and advanced age all are predictors of poor outcome64.
Guillain-Barré syndrome disability scale65
| Score | Description |
| 0 | Healthy state |
| 1 | Minor symptoms and capable of running |
| 2 | Able to walk 10m or more without assistance but unable to run |
| 3 | Able to walk 10m across an open space with help |
| 4 | Bedridden or chair bound |
| 5 | Requiring assisted ventilation for at least part of the day |
| 6 | Dead |
Erasmus GBS Outcome Score (EGOS) is one of the validated prognostic models that have been developed to be used in clinical practice for the prediction of long-term outcome in individual patients with GBS which is based on three clinical characteristics which are age, preceding diarrhea, and a GBS Disability Scale score at 2 weeks after hospital admission.
On the other hand, the modified EGOS can be used to predict the early outcomes in the course of GBS, before irreversible damage occurs, and this model can be used at admission and after admission by 1 week, and it is based on age, the severity of weakness (expressed as MRC sum score), and preceding diarrhea66.
GBS mortality varies between 3% and 7%67. There are Predictors for an increased risk of death and they are advanced age, having severe disease, increased comorbidity, respiratory and cardiac complications, mechanical ventilation, and systemic infection68. Death can occur in any phase of the disease but, there are two studies, that showed a large proportion of the deaths occurred >30 days from onset, and another study showed that the majority of patients who died were in the recovery phase69.
Recent updates and Ongoing researches
There was a new trial in 2014 of a randomized placebo-controlled trial of eculizumab in patients with an early course of GBS who are unable to walk is about to start70, it is based on findings in an animal model of GBS which are mice, in which antiganglioside antibodies induce complement-dependent damage at the nodes of Ranvier, nerve terminals, and peri-synaptic Schwann cells71 72.
These deleterious effects of complement on nerve terminals in mice could be blocked by the administration of (eculizumab), a humanized monoclonal antibody that binds with high affinity to complement factor C5 and prevents its cleavage to C5a and the pro-inflammatory, cytolytic C5b-9 complex.
There are many other studies, such as those investigating pain treatment, nerve regeneration, and other factors that may improve the outcomes of patients with GBS, which are awaited.
The most challenging needs we should work on is to develop improved diagnostic criteria for use in daily clinical practice, trials, and vaccine safety studies, and most importantly, is to develop a more effective and specific treatment also with protocols for supportive care63.
References
- Guillain G, Barré JA, Strohl A. [Radiculoneuritis syndrome with hyperalbuminosis of cerebrospinal fluid without cellular reaction. Notes on clinical features and graphs of tendon reflexes. 1916]. Ann Med Interne (Paris). 1999;150(1):24-32.
- Han ES, goleman, daniel; boyatzis, Richard; Mckee A. Gullian-barre syndrome. Journal of Chemical Information and Modeling. Published 2019. https://emedicine.medscape.com/article/315632-overview
- Wijdicks EFM, Klein CJ. Guillain-Barré Syndrome. Mayo Clin Proc. 2017;92(3):467-479. doi:10.1016/j.mayocp.2016.12.002
- Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet (London, England). 2016;388(10045):717-727. doi:10.1016/S0140-6736(16)00339-1
- Prevots DR, Sutter RW. Assessment of Guillain-Barré syndrome mortality and morbidity in the United States: implications for acute flaccid paralysis surveillance. J Infect Dis. 1997;175 Suppl:S151-5. doi:10.1093/infdis/175.supplement_1.s151
- Esposito S, Longo MR. Guillain-Barré syndrome. Autoimmun Rev. 2017;16(1):96-101. doi:10.1016/j.autrev.2016.09.022
- Zochodne DW. Autonomic involvement in Guillain-Barré syndrome: a review. Muscle Nerve. 1994;17(10):1145-1155. doi:10.1002/mus.880171004
- Maher J, Rutledge F, Remtulla H, Parkes A, Bernardi L, Bolton CF. Neuromuscular disorders associated with failure to wean from the ventilator. Intensive Care Med. 1995;21(9):737-743. doi:10.1007/BF01704741
- Asbury AK. New concepts of Guillain-Barré syndrome. J Child Neurol. 2000;15(3):183-191. doi:10.1177/088307380001500308
- Malek E, Salameh J. Guillain-Barre Syndrome. Semin Neurol. 2019;39(5):589-595. doi:10.1055/s-0039-1693005
- Jacobs BC, Koga M, van Rijs W, et al. Subclass IgG to motor gangliosides related to infection and clinical course in Guillain-Barré syndrome. J Neuroimmunol. 2008;194(1-2):181-190. doi:10.1016/j.jneuroim.2007.11.017
- Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27 Suppl:S21-4. doi:10.1002/ana.410270707
- Hughes RAC, Swan A V, Raphaël J-C, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. doi:10.1093/brain/awm004
- McKhann GM, Cornblath DR, Griffin JW, et al. Acute motor axonal neuropathy: a frequent cause of acute flaccid paralysis in China. Ann Neurol. 1993;33(4):333-342. doi:10.1002/ana.410330402
- Griffin JW, Li CY, Ho TW, et al. Pathology of the motor-sensory axonal Guillain-Barré syndrome. Ann Neurol. 1996;39(1):17-28. doi:10.1002/ana.410390105
- Ho TW, Mishu B, Li CY, et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118 ( Pt 3:597-605. doi:10.1093/brain/118.3.597
- FISHER M. An unusual variant of acute idiopathic polyneuritis (syndrome of ophthalmoplegia, ataxia and areflexia). N Engl J Med. 1956;255(2):57-65. doi:10.1056/NEJM195607122550201
- Overell JR, Hsieh ST, Odaka M, Yuki N, Willison HJ. Treatment for Fisher syndrome, Bickerstaff’s brainstem encephalitis and related disorders. Cochrane database Syst Rev. 2007;(1):CD004761. doi:10.1002/14651858.CD004761.pub2
- Ito M, Kuwabara S, Odaka M, et al. Bickerstaff’s brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. 2008;255(5):674-682. doi:10.1007/s00415-008-0775-0
- Yuki N, Sato S, Tsuji S, Hozumi I, Miyatake T. An immunologic abnormality common to Bickerstaff’s brain stem encephalitis and Fisher’s syndrome. J Neurol Sci. 1993;118(1):83-87. doi:10.1016/0022-510x(93)90250-3
- Department of Neurology, Erasmus University Medical Center, Rotterdam N. variants of GBS. 2019 Sep 20. Published 2019. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6821638/figure/Fig2/
- Pithadia AB, Kakadia N. Guillain-Barré syndrome (GBS). Pharmacol Rep. 2010;62(2):220-232. doi:10.1016/s1734-1140(10)70261-9
- Hahn AF. Guillain-Barré syndrome. Lancet (London, England). 1998;352(9128):635-641. doi:10.1016/S0140-6736(97)12308-X
- van den Berg B, Fokke C, Drenthen J, van Doorn PA, Jacobs BC. Paraparetic Guillain-Barré syndrome. Neurology. 2014;82(22):1984-1989. doi:10.1212/WNL.0000000000000481
- Michael T Andary, MD, MS Professor, Residency Program Director, Department of Physical Medicine and Rehabilitation MSUC of OM. Gullian barre syndrome clinical manifestations. https://emedicine.medscape.com/article/315632-clinical#b3
- Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. doi:10.1016/j.ncl.2013.01.005
- Singhal V, Bhat KG. Guillain-Barre syndrome with hyperreflexia: A variant. J Pediatr Neurosci. 2011;6(2):144-145. doi:10.4103/1817-1745.92844
- Barohn RJ, Saperstein DS. Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy. Semin Neurol. 1998;18(1):49-61. doi:10.1055/s-2008-1040861
- Ruts L, Drenthen J, Jongen JLM, et al. Pain in Guillain-Barre syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. doi:10.1212/WNL.0b013e3181f88345
- autonomic changes in GBS. https://emedicine.medscape.com/article/315632-clinical
- Wang Y, Shang P, Xin M, Bai J, Zhou C, Zhang H-L. The usefulness of chief complaints to predict severity, ventilator dependence, treatment option, and short-term outcome of patients with Guillain-Barré syndrome: a retrospective study. BMC Neurol. 2017;17(1):200. doi:10.1186/s12883-017-0982-3
- gbs physical examination. https://emedicine.medscape.com/article/315632-clinical#b3
- Nguyen TP, Taylor RS. Guillain Barre Syndrome. In: ; 2020.
- Orlikowski D, Prigent H, Sharshar T, Lofaso F, Raphael JC. Respiratory dysfunction in Guillain-Barré Syndrome. Neurocrit Care. 2004;1(4):415-422. doi:10.1385/NCC:1:4:415
- Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33-43. doi:10.1093/brain/awt285
- GBS workup. https://emedicine.medscape.com/article/315632-workup#c9
- Zafar MSH, Naqash MM, Bhat TA, Malik GM. Guillain-barré syndrome in pregnancy: an unusual case. J Fam Med Prim care. 2013;2(1):90-91. doi:10.4103/2249-4863.109965
- Brown WF, Feasby TE. Conduction block and denervation in Guillain-Barré polyneuropathy. Brain. 1984;107 ( Pt 1:219-239. doi:10.1093/brain/107.1.219
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125(Pt 12):2591-2625. doi:10.1093/brain/awf272
- Ang CW, Yuki N, Jacobs BC, et al. Rapidly progressive, predominantly motor Guillain-Barré syndrome with anti-GalNAc-GD1a antibodies. Neurology. 1999;53(9):2122-2127. doi:10.1212/wnl.53.9.2122
- Chiba A, Kusunoki S, Shimizu T, Kanazawa I. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol. 1992;31(6):677-679. doi:10.1002/ana.410310619
- Teitelbaum JS, Borel CO. Respiratory dysfunction in Guillain-Barré syndrome. Clin Chest Med. 1994;15(4):705-714.
- PFT FOR GBS. https://emedicine.medscape.com/article/315632-workup#c12
- Byun WM, Park WK, Park BH, Ahn SH, Hwang MS, Chang JC. Guillain-Barré syndrome: MR imaging findings of the spine in eight patients. Radiology. 1998;208(1):137-141. doi:10.1148/radiology.208.1.9646804
- Jacobs BC, Rothbarth PH, van der Meché FG, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51(4):1110-1115. doi:10.1212/wnl.51.4.1110
- Mishu B, Ilyas AA, Koski CL, et al. Serologic evidence of previous Campylobacter jejuni infection in patients with the Guillain-Barré syndrome. Ann Intern Med. 1993;118(12):947-953. doi:10.7326/0003-4819-118-12-199306150-00006
- Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. Published online December 2020. doi:10.1093/brain/awaa433
- Langmuir AD, Bregman DJ, Kurland LT, Nathanson N, Victor M. An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccines. Am J Epidemiol. 1984;119(6):841-879. doi:10.1093/oxfordjournals.aje.a113809
- de Vries JM, Hagemans MLC, Bussmann JBJ, van der Ploeg AT, van Doorn PA. Fatigue in neuromuscular disorders: focus on Guillain-Barré syndrome and Pompe disease. Cell Mol Life Sci. 2010;67(5):701-713. doi:10.1007/s00018-009-0184-2
- Mx of GBS. https://emedicine.medscape.com/article/315632-treatment#d8
- Arcila-Londono X, Lewis RA. Guillain-Barré syndrome. Semin Neurol. 2012;32(3):179-186. doi:10.1055/s-0032-1329196
- Hughes RA, Rees JH. Clinical and epidemiologic features of Guillain-Barré syndrome. J Infect Dis. 1997;176 Suppl:S92-8. doi:10.1086/513793
- Donofrio PD. Guillain-Barré Syndrome. Continuum (Minneap Minn). 2017;23(5, Peripheral Nerve and Motor Neuron Disorders):1295-1309. doi:10.1212/CON.0000000000000513
- Vitaliti G, Tabatabaie O, Matin N, et al. The usefulness of immunotherapy in pediatric neurodegenerative disorders: A systematic review of literature data. Hum Vaccin Immunother. 2015;11(12):2749-2763. doi:10.1080/21645515.2015.1061161
- van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7(10):939-950. doi:10.1016/S1474-4422(08)70215-1
- Madaan P, Jauhari P, Shruthi NM, Chakrabarty B, Gulati S. Intravenous Immunoglobulin for Severe Protracted Pediatric Guillain-Barre Syndrome: Is Single Dose Adequate? Ann Indian Acad Neurol. 2019;22(1):123-124. doi:10.4103/aian.AIAN_100_18
- van der Meché FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. Dutch Guillain-Barré Study Group. N Engl J Med. 1992;326(17):1123-1129. doi:10.1056/NEJM199204233261705
- Raphaël JC, Chevret S, Hughes RAC, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane database Syst Rev. 2012;(7):CD001798. doi:10.1002/14651858.CD001798.pub2
- Ortiz-Salas P, Velez-Van-Meerbeke A, Galvis-Gomez CA, Rodriguez Q JH. Human Immunoglobulin Versus Plasmapheresis in Guillain-Barre Syndrome and Myasthenia Gravis: A Meta-Analysis. J Clin Neuromuscul Dis. 2016;18(1):1-11. doi:10.1097/CND.0000000000000119
- Sharshar T, Chevret S, Bourdain F, Raphaël J-C. Early predictors of mechanical ventilation in Guillain-Barré syndrome. Crit Care Med. 2003;31(1):278-283. doi:10.1097/00003246-200301000-00044
- Durand M-C, Porcher R, Orlikowski D, et al. Clinical and electrophysiological predictors of respiratory failure in Guillain-Barré syndrome: a prospective study. Lancet Neurol. 2006;5(12):1021-1028. doi:10.1016/S1474-4422(06)70603-2
- Walgaard C, Lingsma HF, Ruts L, et al. Prediction of respiratory insufficiency in Guillain-Barré syndrome. Ann Neurol. 2010;67(6):781-787. doi:10.1002/ana.21976
- Cheng B-C, Chang W-N, Chen J-B, et al. Long-term prognosis for Guillain-Barré syndrome: evaluation of prognostic factors and clinical experience of automated double filtration plasmapheresis. J Clin Apher. 2003;18(4):175-180. doi:10.1002/jca.10066
- Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333(21):1374-1379. doi:10.1056/NEJM199511233332102
- van Koningsveld R, Steyerberg EW, Hughes RAC, Swan A V, van Doorn PA, Jacobs BC. A clinical prognostic scoring system for Guillain-Barré syndrome. Lancet Neurol. 2007;6(7):589-594. doi:10.1016/S1474-4422(07)70130-8
- Walgaard C, Lingsma HF, Ruts L, van Doorn PA, Steyerberg EW, Jacobs BC. Early recognition of poor prognosis in Guillain-Barre syndrome. Neurology. 2011;76(11):968-975. doi:10.1212/WNL.0b013e3182104407
- Alshekhlee A, Hussain Z, Sultan B, Katirji B. Guillain-Barré syndrome: incidence and mortality rates in US hospitals. Neurology. 2008;70(18):1608-1613. doi:10.1212/01.wnl.0000310983.38724.d4
- Lawn ND, Wijdicks EF. Fatal Guillain-Barré syndrome. Neurology. 1999;52(3):635-638. doi:10.1212/wnl.52.3.635
- van den Berg B, Bunschoten C, van Doorn PA, Jacobs BC. Mortality in Guillain-Barre syndrome. Neurology. 2013;80(18):1650-1654. doi:10.1212/WNL.0b013e3182904fcc
- NHS Greater Glasgow and Clyde. Inhibition of Complement Activation (Eculizumab) in Guillain-Barre Syndrome Study (ICA-GBS). Published online 2014. https://www.clinicaltrials.gov/ct2/show/NCT02029378
- Susuki K, Rasband MN, Tohyama K, et al. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J Neurosci. 2007;27(15):3956-3967. doi:10.1523/JNEUROSCI.4401-06.2007
- Plomp JJ, Willison HJ. Pathophysiological actions of neuropathy-related anti-ganglioside antibodies at the neuromuscular junction. J Physiol. 2009;587(Pt 16):3979-3999. doi:10.1113/jphysiol.2009.171702





I found this article very informative and pleasant to read. It’s everyday we unlock a new mystery in the plexus of our nervous system, quite marvelous.
Thank you so much
The condition presentation is objective, comprehensive and full of important details.
Well done Dr. Abdallah!
This article provides great insight into the theoretical and clinical aspects of GBS, it’s a great read. Very well done!