Article Topic: Alzheimer’s Disease
Name of the Author: Leen Sawalha
Key Words: Alzheimer’s disease, AD, Dementia, Amyloid Beta, Tau, Neurodegenerative Diseases
Overview and Epidemiology:
Dementia is a cognitive degenerative disease that has affected 44 million people around the world, with predictions that this number will triple by the year 2050 (1). Out of all dementias, Alzheimer’s is the most common. It mainly affects elderly people, with the occurrence increasing by 15 times beyond the age of 65 (2).
Having Alzheimer’s below the age of 65 is scarce. However, if it is present at those earlier ages, it is known as early onset Alzheimer’s disease (3). It has been estimated by the CDC (Centers for Disease Control and Prevention) that the number of those who surpass the age of 65 will rise from 420 million to 1 billion people between the years of 2000 to 2030; hence, markedly increasing the susceptible target population of the disease (4).
Paradoxically, although it is believed that Alzheimer’s will continue to increase in number, another study shows that the cases have decreased by 24% between 2000-2012 due to an increase in the levels of education seen in those years (5).
Pathogenesis and Etiology:
It is hypothesized that cholinergic dysfunction could cause Alzheimer’s (6). Patients with the disease have been reported to have a reduced level of activity in choline acetyltransferase compared to those who do not have Alzheimer’s (7). A decrease in the number of nicotinic receptors along with muscarinic acetyl choline receptors in the presynaptic terminal contributes to a decrease in cognitive ability of the individual (8).
The most important genetic factor that plays a role in the pathogenesis of late onset Alzheimer’s is the presence of the APOE ɛ4 allele. This gene codes for apolipoprotein E which is abundant in the brain and is thought to promote the accumulation of amyloid beta and tau proteins inside the brain (15).
Furthermore, inflammation in the brain, which is shown to have an initial protective function, will have deleterious effects on the progression of the disease when a chronic response is activated. This happens because microglial cells release many toxic products that harm the brain (16).
Clinical Presentation and Complications:
There are 10 early symptoms that should be looked out for. These include :
significant memory loss, struggling in making plans, inability to use appliances and devices, forgetting locations, difficulty processing visuals, difficulty engaging in conversations, misplacing items, diminished ability to judge wisely, avoidance of social interactions and lastly, anxiety and depression (18).
The later symptoms of Alzheimer’s manifest both behaviorally and psychiatrically. Psychiatric symptoms include depression. A study suggests that the coexistence of Alzheimer’s along with depression results in much worse clinical outcomes for patients (19). Apathy, which is defined as the loss of emotion and aimless cognition, is also a symptom of Alzheimer’s. Its presence is indicative of swifter cognitive diminishment (20)(21). Agitation and aggression are also seen in patients (21). This poses a significant problem since this has been shown to correlate with an inability to be independent (22). Agitation is seen more commonly in male patients than in female patients (23). Furthermore, psychosis is also seen in those suffering from Alzheimer’s and is considered to be the most profound yet least common complication in the early stages (24). As mentioned previously about depression, the presence of psychosis also contributes to an accelerated decrease in cognitive functional ability and an increase in mortality rate (25)(26)(27).
A study found that the most recurrent complications associated with Alzheimer’s are patients going astray, stumbling, pneumonia, and fractured bones. The study also concluded that males are more likely to get lost and that a lower level of education was a risk factor for acquiring pneumonia (28). Another prominent complication of Alzheimer’s is dysphagia which subsequently leads to multiple problems such as malnutrition, dehydration and loss of weight, all of which severely impact the quality of life of the individual and decrease it drastically (29).
Workup and Diagnosis:
The preclinical stage of Alzheimer’s is unnoticeable with barely any symptoms. Hence, it is difficult to diagnose early on. However, for a diagnosis to be made, cognitive decline must be extremely evident and must reach a point where it interferes with daily activities (30).
The National Institute of Aging-Alzheimer’s Association (NIA-AA) criteria is the currently used criteria for diagnosis of the disease. These guidelines recognize that the beginning of AD occur long before symptoms are evident.
There are 3 stages:
- Preclinical AD, in which biomarkers of AD may be present but no symptoms.
- MCI: Mild cognitive symptoms without significant functional impairment.
- Dementia due to AD.
Mild cognitive impairment is a mental condition in which the symptoms observed are between those seen in normal aging and those seen in dementia. It can sometimes be a predecessor to probable AD. The cognitive state observed at this stage does not significantly impair day to day activities. Memory impediment is also observed (33). In some circumstances, mild cognitive impairment is caused by treatable precursors. Examples include MCI that is acquired due to psychiatric diseases and concurrent illnesses. The cognitive diminishment seen here can be reversed. It should be noted that mild cognitive impairment is not necessarily indicative of Alzheimer’s disease. Moreover, there are no treatments approved for MCI by the FDA (34)
Diagnosing Alzheimer’s Disease:
The NIA-AA divides Alzheimer’s into possible and probable AD. To be diagnosed with probable AD:
- The patient must meet the criteria for dementia which includes:
a. Inability to function in daily life.
b. A decrease in performance
c. Symptoms that are inexplicable by other psychiatric disorders like delirium,
d. Cognitive decline which is diagnosed by taking history and cognitive assessment.
e. In addition to 2 of the following symptoms: inability to remember new information, hindered reasoning, diminished visuospatial skills, decrease in language skills and/or changes in personality (35).
2. Affected by insidious onset, declining cognitive ability and conspicuous cognitive deficits in tests done for one of the following amnestic symptoms: learning and recall deficits with impaired functioning ability in at least one other cognitive aspect. Additionally, they must have non-amnestic symptoms such as being unable to recall and string together words, diminished visuospatial ability and executive malfunction.
3. A diagnosis should not be made when these symptoms are accompanied by cerebrovascular disease, characteristics of dementia with Lewy bodies, behavioral characteristics of frontotemporal dementia, properties of semantic variant primary progressive aphasia or non-fluent or agrammatic variant primary progressive aphasia, the coexistence of another neurological/non-neurological disease or the use of drugs that affect cognitive functional ability (35).
Diagnosing possible dementia requires that the patient has an unusual course or an etiologically varied presentation (36).
Neuroimaging:
1.On MRI, cortical atrophy is apparent. This has been attributed to the presence of neurofibrillary tangles, thus, it is considered to be associated with Alzheimer’s (38). Additionally, atrophy of structures found in the mesial temporal lobe, such as the hippocampus and entorhinal cortex is also evident (39). Moreover, the presence of periventricular white matter hyperintensities are indicative of development of mild cognitive impairment into AD (40). A substantial decrease in the size of the temporoparietal cortex is also seen on MRI and is a marker for the presence of the disease (41). Ventricular enlargement is also apparent on MRI and can be attributed to mild cognitive impairment and AD (42)(43)(44)(45)(46).
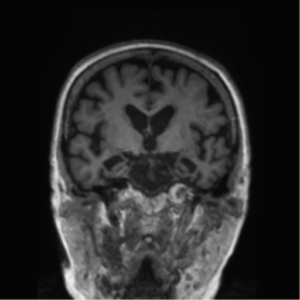
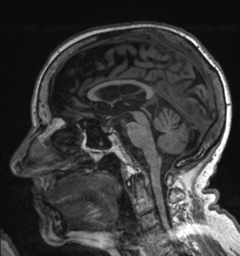
Figure 1: Alzheimer’s Disease, Case courtesy of Dr Bruno Di Muzio (47)
2. Functional brain imaging (FDG-PET, SPECT): The temporoparietal cortex and posterior cingulate/precuneus reveals distinct regions of hypometabolism (FDG-PET) or hypoperfusion (SPECT), useful for differentiating AD from FTD (predominant hypometabolism in frontal and anterior temporal regions).
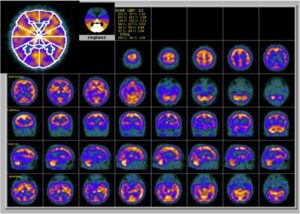
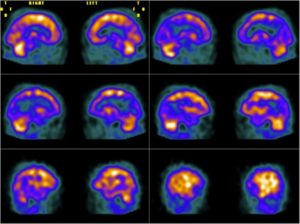
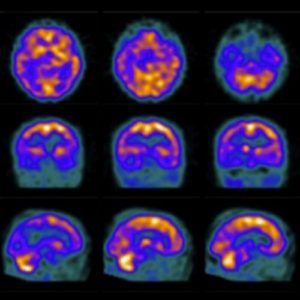
Figure 2: Brain perfusion with 99mTc-ECD shows bilateral temporoparietal hypoperfusion (48).
Neuropathology:
On a neuropathological scale, AD is defined by the presence of two lesions:
- Senile Amyloid plaques
- Tau neurofibrillary tangles
Alzheimer’s is known to be the accumulation of tau proteins forming neurofibrillary lesions (9)(10). Tau proteins are microtubule associated proteins that are found inside the neurons of the brain (11). Taking all this into account, the tau hypothesis states that abnormal phosphorylation of the protein leads to a change from normal tau into PHF tau (12).
Moreover, we have the amyloid cascade hypothesis. This hypothesis states that accumulation of amyloid beta (Aβ) inside the brain can result in neurodegeneration which can contribute to Alzheimer’s (13)(14).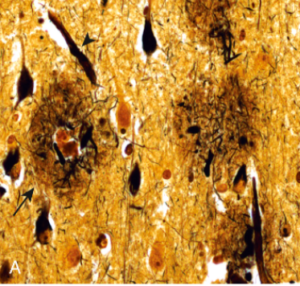
Figure 3: Plaques (arrow) contain a central core of amyloid and a surrounding region of dystrophic neurites (Bielschowsky stain) (17).
Figure 4: Immunohistochemical stain for Aβ. Peptide is present in the core of the plaques as well as in the surrounding region (17).
Figure 5: Neurons containing tangles stained with an antibody specific for tau (17).
Biomarkers:
Biomarkers of amyloid, tau, and neurodegeneration proposed in National Institute of Aging-Alzheimer’s Association (NIA-AA) research framework for defining AD (TABLE 1):
TABLE 1. AT(N) biomarker grouping (49).
| A: Aggregated Aβ or associated pathologic state · CSF Aβ42, or Aβ42/Aβ40 ratio · Amyloid PET |
| T: Aggregated tau (neurofibrillary tangles) or associated pathologic state · CSF phosphorylated tau · Tau PET |
| (N): Neurodegeneration or neuronal injury · Anatomic MRI · FDG PET · CSF total tau |
Abbreviations: Aβ, β amyloid; CSF, cerebrospinal fluid.
Additionally, a test called ‘Mini Mental State Examination’ is used to screen for dementia. It tests a range of cognitive functions such as attention, memory, language, visuoconstruction and orientation. The maximum score that could be obtained is 30. Scoring 23 and less is considered to signify impairment. Every year, those with Alzheimer’s score 3 points less than they did the previous year. This is used as an adjunct diagnosis technique; it is never used by itself to make a diagnosis. It very useful in determining the severity of dementia (37).
Treatment and Management:
Psychosocial treatment options for Alzheimer’s include changing the surrounding environment of the patient and aid from family members. There is evidence to suggest that this improves the wellbeing of those suffering from the disease (50)(51).
Many of the treatments offered nowadays only provide symptomatic relief rather than reverse the disease progression (52).
Pharmacological treatments include:
- Acetyl cholinesterase inhibitors such as donepezil, galantamine and rivastigmine. They are all approved for the treatment of Alzheimer’s and work by inhibiting the enzyme acetylcholinesterase (53)(54)(55). Acetylcholine is in charge of memory and learning, which would explain the therapeutic effect increasing its amount has on Alzheimer’s (56).
- Memantine, which is an N-methyl-D-aspartate receptor antagonist, is also used as a treatment option. Glutamate increases the amount of calcium inside the cells and leads to a malfunction in the mitochondria resulting in an increased production of oxidants. Therefore, a destruction of neurons will occur. This increase in stimulation can be counteracted by NMDA receptor antagonists (57)(58).
Some studies suggest that NSAIDs could help in the treatment of Alzheimer’s as transgenic animals that were given those drugs showed a reduction in the pathology of the disease (59). Unfortunately, when applied to humans, no evidence of efficacy has been observed in the trials conducted (60).
For behavioral disturbances antipsychotics, anxiolytics, mood stabilizers and hypnotics can be used to treat the associated symptoms (52).
To treat depression that accompanies Alzheimer’s tricyclic antidepressants like nortriptyline and desipramine can be used. They show less anticholinergic activity than their parent compounds (61). Additionally, reversible monoamine oxidase inhibitors such as brofaromine and moclobemide, can be used in the treatment of depression related to Alzheimer’s (62).
Furthermore, vitamin E, which is an antioxidant, has been suggested as a possible choice for prophylaxis against the disease. A study conducted showed that those who took vitamin E had decelerated progression to Alzheimer’s. The same study indicated that the use of selegiline, which is a drug that increases the release of dopamine, is also beneficial in slowing down Alzheimer’s (63)(64).
Risk Factors:
- The APOE gene presents a notable increase in the risk of the development of dementia. This was evident in a study conducted in 1993 which stated that as the amount of APOEe4 alleles escalates, the risk of early onset Alzheimer’s grows from a mere 20% to a staggering 90% risk (65).
- Gender has also been attributed as a risk factor for the development of Alzheimer’s. A study has shown that females are more likely to suffer from Alzheimer’s after 85 years of age (66).
- A research paper suggests that being overweight can lead to Alzheimer’s since obesity is also linked to diabetes and hyperinsulinemia which are both risk factors for the development of the disease (67).
- Age is also a risk factor. An increase in age increased the probability of Alzheimer’s as shown in studies conducted on many areas around the world and included nationalities such as European, Chinese, American and Spanish (68)(69)(70)(71).
- A study conducted on men 50 and older concluded that those who suffered from sleep disturbances had a 51% increased risk of developing Alzheimer’s (72).
- A French study concluded that there is a possible risk of users of benzodiazepines acquiring Alzheimer’s. However, more research needs to be done in order for a solid correlation to be made (73).
- Brain injury has also been suggested to increase the risk of acquiring the disease since the prevalence of brain injury and Alzheimer’s has increased concurrently (74). It is thought that the link is due to the rise in amount of amyloid beta within the brain a few weeks after head trauma. This can be attributed to a release of interleukin 1 which increases the deposition of amyloid-beta precursor protein (APP) and amyloid beta (75)(76).
Prevention:
There are multiple preventative measures that could be taken.
- A study found that those with higher blood pressure were more likely to develop Alzheimer’s (77). In elderly patients suffering from hypertension, the intake of antihypertensives helped conserve cognitive functional ability and decreased the risk of dysfunction by 38% (78).
- Elevated levels of cholesterol have also been associated with Alzheimer’s (79). Hence, statins, which are drugs that reduce cholesterol levels, are used to defend against the disease (80).
- Engaging in leisure activities that stimulate mental function, such as reading a newspaper, learning, playing card games or arts and crafts, has also shown to lower the risk of Alzheimer’s (81)(82)(83). Additionally, physical exercise is suggested to have a preventative role against development of the disease since it increases cognitive functional ability (84)(85).
- Diet also plays a role in the risk of AD. An increase in ingestion of saturated and hydrogenated fats showed an association with a higher risk of Alzheimer’s, while as the consumption of monounsaturated and polyunsaturated fats has been shown to help prevent mental decline (86)(87)(88).
Prognosis:
A study conducted to test the prognosis of Alzheimer’s concluded that those suffering from AD had a high incidence of institutionalization and death. Additionally, they found that 11.84% of those with the disease experienced swift cognitive functional diminishment. Moreover, the cohort study showed that 2/3 of those participating had a notable evolution of Alzheimer’s (89).
Another study showed that after a 5-year study period, those participating had an 80% dependence on others in their daily activities. 75% of those followed got institutionalized. Additionally, the percentage of incontinence among participants was 74%. After 4 years, most of the patients being studied showed incontinence or dependence in activities of daily living (90).
Furthermore, another study found that those suffering from probable AD had a 3.1 year median survival rate and 3.5 years for possible Alzheimer’s (91).
Recent Updates:
Stem cell therapy shows tremendous potential for the treatment of Alzheimer’s. It has been shown to be effective in the treatment of transgenic rodents. However, human trials have still not showed any evidence of efficacy. This may be due to the presence of many factors that govern the disease and due to the limitations of the studies conducted. Hence, more information along with more studies are required to achieve substantial results (92).
Moreover, recent trials have shown a new monoclonal antibody, known as Aducanumab, to be promising. This drug targets amyloid beta and removes the cluster that accumulated, thus, decelerating the decline in mental capacity (93).
References:
-
- Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018 Jan;25(1):59–70.
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, et al. Prevalence of Alzheimer’s Disease in a Community Population of Older Persons: Higher Than Previously Reported. JAMA. 1989 Nov;262(18):2551–6.
- Erkkinen MG, Kim M-O, Geschwind MD. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2018 Apr;10(4).
- Public Health and Aging: Trends in Aging—United States and Worldwide. JAMA. 2003 Mar;289(11):1371–3.
- Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017 Jan;177(1):51–8.
- Contestabile A. The history of the cholinergic hypothesis. Vol. 221, Behavioural Brain Research. Elsevier; 2011. p. 334–40.
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Vol. 2, Lancet (London, England). England; 1976. p. 1403.
- Nordberg A, Alafuzoff I, Winblad B. Nicotinic and muscarinic subtypes in the human brain: Changes with aging and dementia. J Neurosci Res. 1992;31(1):103–11.
- Forman MS, Trojanowski JQ, Lee VM-Y. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10(10):1055–63.
- Trojanowski JQ, Mattson MP. Overview of protein aggregation in single, double, and triple neurodegenerative brain amyloidoses. NeuroMolecular Med. 2003;4(1):1–5.
- Tucker RP. The roles of microtubule-associated proteins in brain morphogenesis: a review. Vol. 15, Brain Research Reviews. Elsevier; 1990. p. 101–20.
- Mohandas E, Rajmohan V, Raghunath B. Neurobiology of Alzheimer’s disease. Indian J Psychiatry. 2009 Jan;51(1):55–61.
- Hardy JA, Higgins GA. Alzheimer’s disease: The amyloid cascade hypothesis. Science (80- ). 1992;256(5054):184–5.
- Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Aβ amyloid peptides. Peptides. 2002;23(7):1285–97.
- Muñoz SS, Garner B, Ooi L. Understanding the Role of ApoE Fragments in Alzheimer’s Disease. Neurochem Res. 2019 Jun;44(6):1297–305.
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006 Aug;38(4):333–47.
- Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology. Tenth Edit. Elsevier; 2018.
- 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013 Mar;9(2):208–45.
- Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2015 Feb;23(2):130–40.
- Ota M, Sato N, Nakata Y, Arima K, Uno M. Relationship between apathy and diffusion tensor imaging metrics of the brain in Alzheimer’s disease. Int J Geriatr Psychiatry. 2012 Jul;27(7):722–6.
- Li X-L, Hu N, Tan M-S, Yu J-T, Tan L. Behavioral and psychological symptoms in Alzheimer’s disease. Biomed Res Int. 2014;2014:927804.
- Bidzan L, Bidzan M, Pąchalska M. Aggressive and impulsive behavior in Alzheimer’s disease and progression of dementia. Med Sci Monit Int Med J Exp Clin Res. 2012 Mar;18(3):CR182-9.
- Kitamura T, Kitamura M, Hino S, Tanaka N, Kurata K. Gender differences in clinical manifestations and outcomes among hospitalized patients with behavioral and psychological symptoms of dementia. J Clin Psychiatry. 2012 Dec;73(12):1548–54.
- Lanctôt KL, Herrmann N, Eryavec G, van Reekum R, Reed K, Naranjo CA. Central serotonergic activity is related to the aggressive behaviors of Alzheimer’s disease. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2002 Oct;27(4):646–54.
- Lee GJ, Lu PH, Hua X, Lee S, Wu S, Nguyen K, et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biol Psychiatry. 2012 May;71(9):814–21.
- Wilkosz PA, Seltman HJ, Devlin B, Weamer EA, Lopez OL, DeKosky ST, et al. Trajectories of cognitive decline in Alzheimer’s disease. Int psychogeriatrics. 2010 Mar;22(2):281–90.
- Gilley DW, Bienias JL, Wilson RS, Bennett DA, Beck TL, Evans DA. Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer’s disease. Psychol Med. 2004 Aug;34(6):1129–35.
- Alhazzani AA, Alqahtani M, Alshbriqe A, Awwadh A, Alyami T, Alshomrani M, et al. Prevalence of complications associated with Alzheimer disease. J Neurol Sci. 2017;381:315.
- Boccardi V, Ruggiero C, Patriti A, Marano L. Diagnostic Assessment and Management of Dysphagia in Patients with Alzheimer’s Disease. J Alzheimers Dis. 2016;50(4):947–55.
- Förstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288–90.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939–44.
- López OL, Dekosky ST. Clinical symptoms in Alzheimer’s disease. Handb Clin Neurol. 2008;89:207–16.
- Petersen RC. Aging, mild cognitive impairment, and Alzheimer’s disease. Neurol Clin. 2000 Nov;18(4):789–806.
- Petersen RC. Mild Cognitive Impairment. Continuum (Minneap Minn). 2016 Apr;22(2 Dementia):404–18.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CRJ, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):263–9.
- Atri A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med Clin North Am. 2019;103(2):263–93.
- Bernard BA, Goldman JG. MMSE – Mini-Mental State Examination. In: Kompoliti K, Metman LV, editors. Encyclopedia of Movement Disorders. Oxford: Academic Press; 2010. p. 187–9.
- Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008 Sep;71(10):743–9.
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004 Mar;25(3):303–10.
- van Straaten ECW, Harvey D, Scheltens P, Barkhof F, Petersen RC, Thal LJ, et al. Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol. 2008 Sep;255(9):1302–8.
- Whitwell JL, Jack CRJ, Przybelski SA, Parisi JE, Senjem ML, Boeve BF, et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2011 Sep;32(9):1531–41.
- Jack CRJ, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004 Feb;62(4):591–600.
- Wang D, Chalk JB, Rose SE, de Zubicaray G, Cowin G, Galloway GJ, et al. MR image-based measurement of rates of change in volumes of brain structures. Part II: application to a study of Alzheimer’s disease and normal aging. Magn Reson Imaging. 2002 Jan;20(1):41–8.
- Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol. 2000 Mar;57(3):339–44.
- Bradley KM, Bydder GM, Budge MM, Hajnal J V, White SJ, Ripley BD, et al. Serial brain MRI at 3-6 month intervals as a surrogate marker for Alzheimer’s disease. Br J Radiol. 2002 Jun;75(894):506–13.
- Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology. 2005 Jul;65(1):119–24.
- Radiopaedia. Alzheimer Disease.
- Radiopaedia. FDG-PET, SPECT. In. Available from: https://radiopaedia.org/cases/alzheimer-disease-spect?lang=gb
- Jack CRJ, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018 Apr;14(4):535–62.
- Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA. 1996 Dec;276(21):1725–31.
- Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997 Mar;277(10):806–12.
- Schachter AS, Davis KL. Alzheimer’s disease. Dialogues Clin Neurosci. 2000 Jun;2(2):91–100.
- Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res. 2013 Apr;36(4):375–99.
- Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. Lancet Neurol. 2015 Sep;14(9):926–44.
- Godyń J, Jończyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep. 2016 Feb;68(1):127–38.
- Mitsushima D, Sano A, Takahashi T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun. 2013;4:2760.
- Prentice H, Modi JP, Wu J-Y. Mechanisms of Neuronal Protection against Excitotoxicity, Endoplasmic Reticulum Stress, and Mitochondrial Dysfunction in Stroke and Neurodegenerative Diseases. Oxid Med Cell Longev. 2015;2015:964518.
- Shi X, Lin X, Hu R, Sun N, Hao J, Gao C. Toxicological Differences Between NMDA Receptor Antagonists and Cholinesterase Inhibitors. Am J Alzheimers Dis Other Demen. 2016 Aug;31(5):405–12.
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007 May;28(5):639–47.
- Miguel-Álvarez M, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Garatachea N, et al. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging. 2015 Feb;32(2):139–47.
- Schweizer E, Rickels K, Hassman H, Garcia-España F. Buspirone and imipramine for the treatment of major depression in the elderly. J Clin Psychiatry. 1998 Apr;59(4):175–83.
- Nair NP, Amin M, Holm P, Katona C, Klitgaard N, Ng Ying Kin NM, et al. Moclobemide and nortriptyline in elderly depressed patients. A randomized, multicentre trial against placebo. J Affect Disord. 1995 Jan;33(1):1–9.
- Ebadi M, Sharma S, Shavali S, El Refaey H. Neuroprotective actions of selegiline. J Neurosci Res. 2002 Feb;67(3):285–9.
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997 Apr;336(17):1216–22.
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993 Aug;261(5123):921–3.
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, et al. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999 Dec;53(9):1992–7.
- Luchsinger JA, Mayeux R. Adiposity and Alzheimer’s disease. Curr Alzheimer Res. 2007 Apr;4(2):127–34.
- Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurology. 2000 Mar;54(5):1109–16.
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999 Jan;52(1):78–84.
- López-Pousa S, Vilalta-Franch J, Llinàs-Regla J, Garre-Olmo J, Román GC. Incidence of dementia in a rural community in Spain: the Girona cohort study. Neuroepidemiology. 2004;23(4):170–7.
- Liu L, Guo X-E, Zhou Y-Q, Xia J-L. Prevalence of dementia in China. Dement Geriatr Cogn Disord. 2003;15(4):226–30.
- Benedict C, Byberg L, Cedernaes J, Hogenkamp PS, Giedratis V, Kilander L, et al. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimers Dement. 2015 Sep;11(9):1090–7.
- Lagnaoui R, Bégaud B, Moore N, Chaslerie A, Fourrier A, Letenneur L, et al. Benzodiazepine use and risk of dementia: a nested case-control study. J Clin Epidemiol. 2002 Mar;55(3):314–8.
- Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer’s disease: a review. Neuropsychol Rev. 2000 Jun;10(2):115–29.
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000 Oct;55(8):1158–66.
- Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, et al. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7606–10.
- Wu C, Zhou D, Wen C, Zhang L, Como P, Qiao Y. Relationship between blood pressure and Alzheimer’s disease in Linxian County, China. Life Sci. 2003 Jan;72(10):1125–33.
- Murray MD, Lane KA, Gao S, Evans RM, Unverzagt FW, Hall KS, et al. Preservation of cognitive function with antihypertensive medications: a longitudinal analysis of a community-based sample of African Americans. Arch Intern Med. 2002 Oct;162(18):2090–6.
- Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J Neurosci Res. 2003 Apr;72(2):141–6.
- Burns M, Duff K. Use of in vivo models to study the role of cholesterol in the etiology of Alzheimer’s disease. Neurochem Res. 2003 Jul;28(7):979–86.
- Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes De Leon CF, Morris MC, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002 Dec;59(12):1910–4.
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002 Feb;287(6):742–8.
- Yates LA, Ziser S, Spector A, Orrell M. Cognitive leisure activities and future risk of cognitive impairment and dementia: systematic review and meta-analysis. Int psychogeriatrics. 2016 Nov;28(11):1791–806.
- Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord. 2004;18(2):57–64.
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004 Oct;85(10):1694–704.
- Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004 Oct;3(10):579–87.
- Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003 Jan;110(1):95–110.
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003 Feb;60(2):194–200.
- Cortes F, Nourhashémi F, Guérin O, Cantet C, Gillette-Guyonnet S, Andrieu S, et al. Prognosis of Alzheimer’s disease today: a two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement. 2008 Jan;4(1):22–9.
- Drachman DA, O’Donnell BF, Lew RA, Swearer JM. The prognosis in Alzheimer’s disease. “How far” rather than “how fast” best predicts the course. Arch Neurol. 1990 Aug;47(8):851–6.
- Wolfson C, Wolfson DB, Asgharian M, M’Lan CE, Ostbye T, Rockwood K, et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med. 2001 Apr;344(15):1111–6.
- Vasic V, Barth K, Schmidt MHH. Neurodegeneration and Neuro-Regeneration-Alzheimer’s Disease and Stem Cell Therapy. Int J Mol Sci. 2019 Aug;20(17).
- Malik GA, Robertson NP. Treatments in Alzheimer’s disease. J Neurol. 2017 Feb;264(2):416–8.